Controlled release of active aldehydes and ketones from equilibrated dynamic mixtures
A mixture, reactive aldehyde technology, applied in animal repellents, organic active ingredients, detergent composition fragrances, etc., can solve the problem of not mentioning the principle of dynamic mixture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
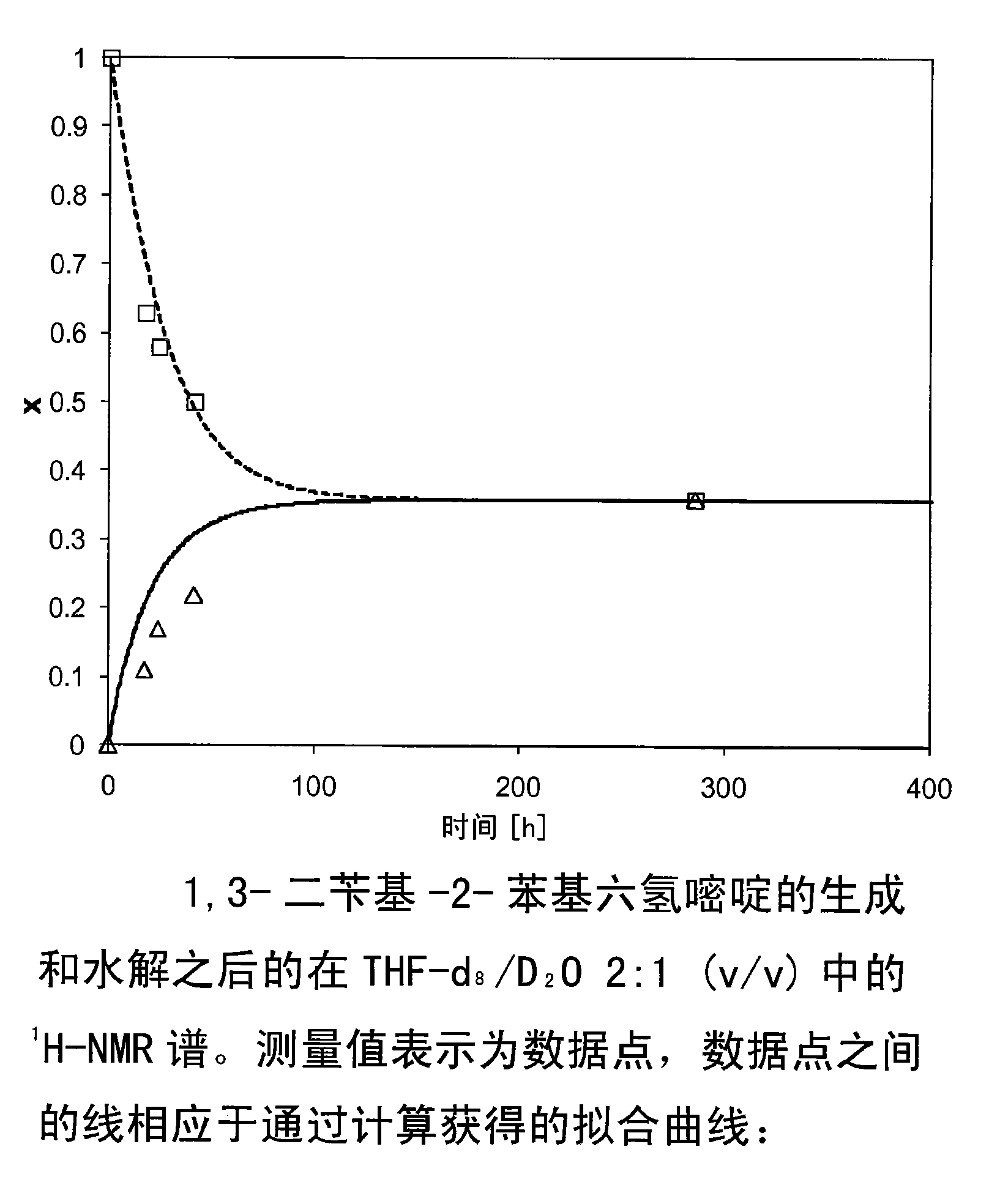
[0232] Generation of the dynamic mixture of the present invention
[0233] In deuterated aqueous buffer solution (DMSO-d 6 / D 2 O 2:1 (v / v)) through 1 H-NMR spectroscopy monitors the formation of kinetic mixtures. The aqueous portion of the deuterated buffer stock was prepared from the following quantities of product:
[0234] Na 2 HPO 4 0.817g
[0235] K H 2 PO 4 0.107g
[0236] D. 2 O 22.10g (= 20ml)
[0237] Add 1.0ml DMSO-d 6 into the aqueous portion of 0.5 ml deuterated buffered stock solution to produce a final reaction solution whose pH was determined to be 6.5-7.0 (using Merck pH test paper 5.5 ~ 9.0).
[0238] In order to verify the generation and hydrolysis of the aminal derivatives of the present invention, the same equilibrium is formed, in DMSO-d 6 180 mM solutions of diamine derivatives, reactive aldehydes or ketones and corresponding aminal derivatives were prepared in . Then, add 0.05 ml of diamine derivative solution, 0.05 ml of reac...
Embodiment 2
[0246] The reversibility of the equilibrium of the dynamic mixture of the present invention
[0247] In order to demonstrate that the same equilibrium is obtained in both directions of the reaction and to determine the corresponding equilibrium constants, after the generation and hydrolysis of the aminals of the present invention, the deuterated aqueous buffer stock solution (THF-d 8 / D 2 O 2:1 (v / v)) 1 H-NMR. The aqueous portion of the deuterated buffer stock was prepared as described above (Example 1).
[0248] For this assay, in THF-d 8 180 mM solutions of diamine derivatives and reactive aldehydes were prepared in . Similarly, 90 mM solutions of the corresponding aminals were prepared in the same solvent. Then, to 0.3 ml of aqueous buffer stock solution in an NMR tube, add 0.05 ml of diamine derivative solution, 0.05 ml of reactive aldehyde solution, and 0.50 ml of THF-d 8 , or add 0.10ml of the corresponding aminal derivative and 0.50ml of THF-d 8 . Thus, each t...
Embodiment 3
[0263] Properties of Softener Bases Comprising Kinetic Mixtures of the Invention
[0264] The mixtures of the invention have been tested for use as perfuming ingredients in fabric softeners. A fabric softener base has been prepared with the following final composition:
[0265] VK90 (source: Stepan) 16.5wt%
[0266] Calcium chloride 0.2wt%
[0267] Water 83.3wt%
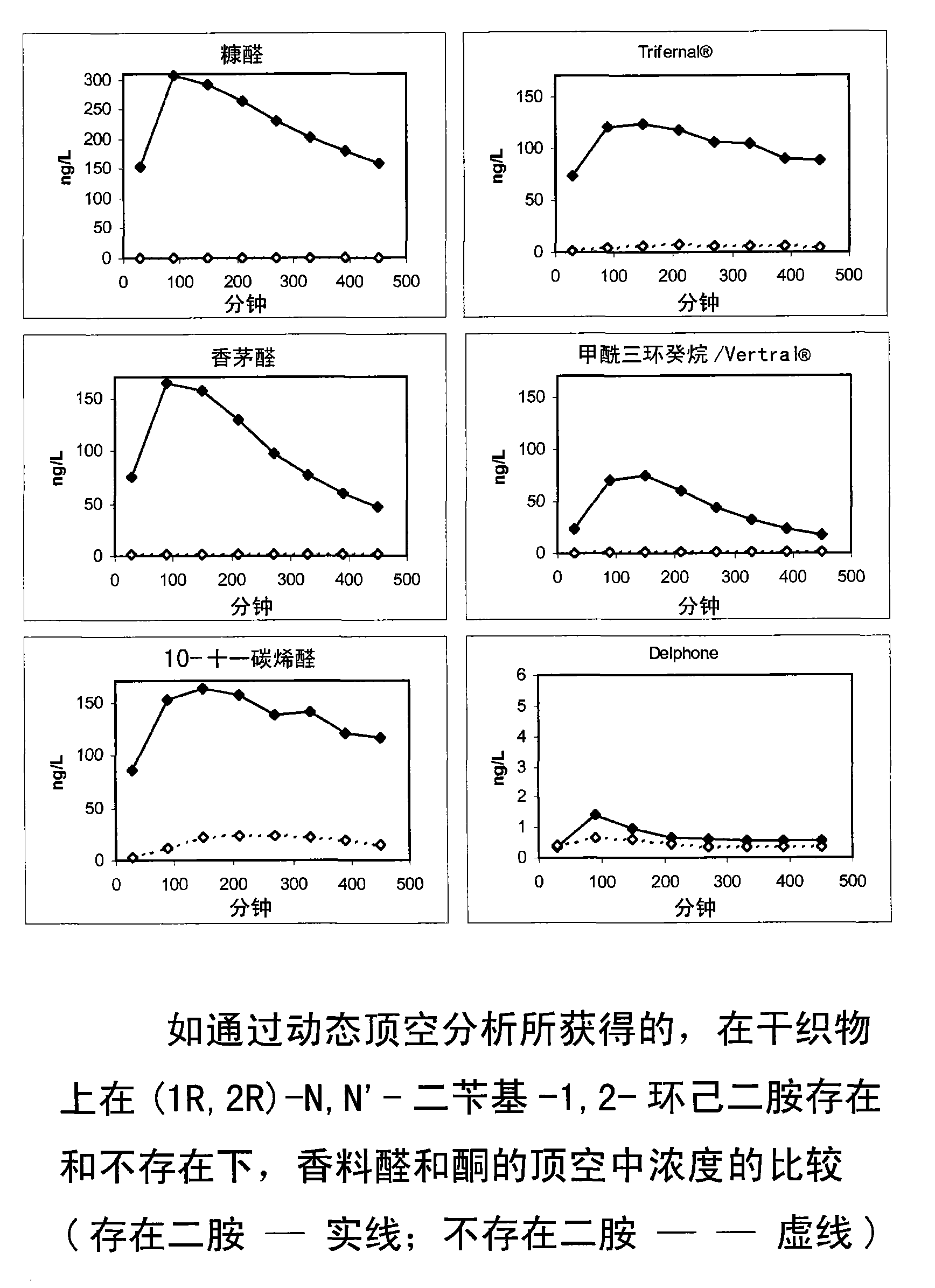
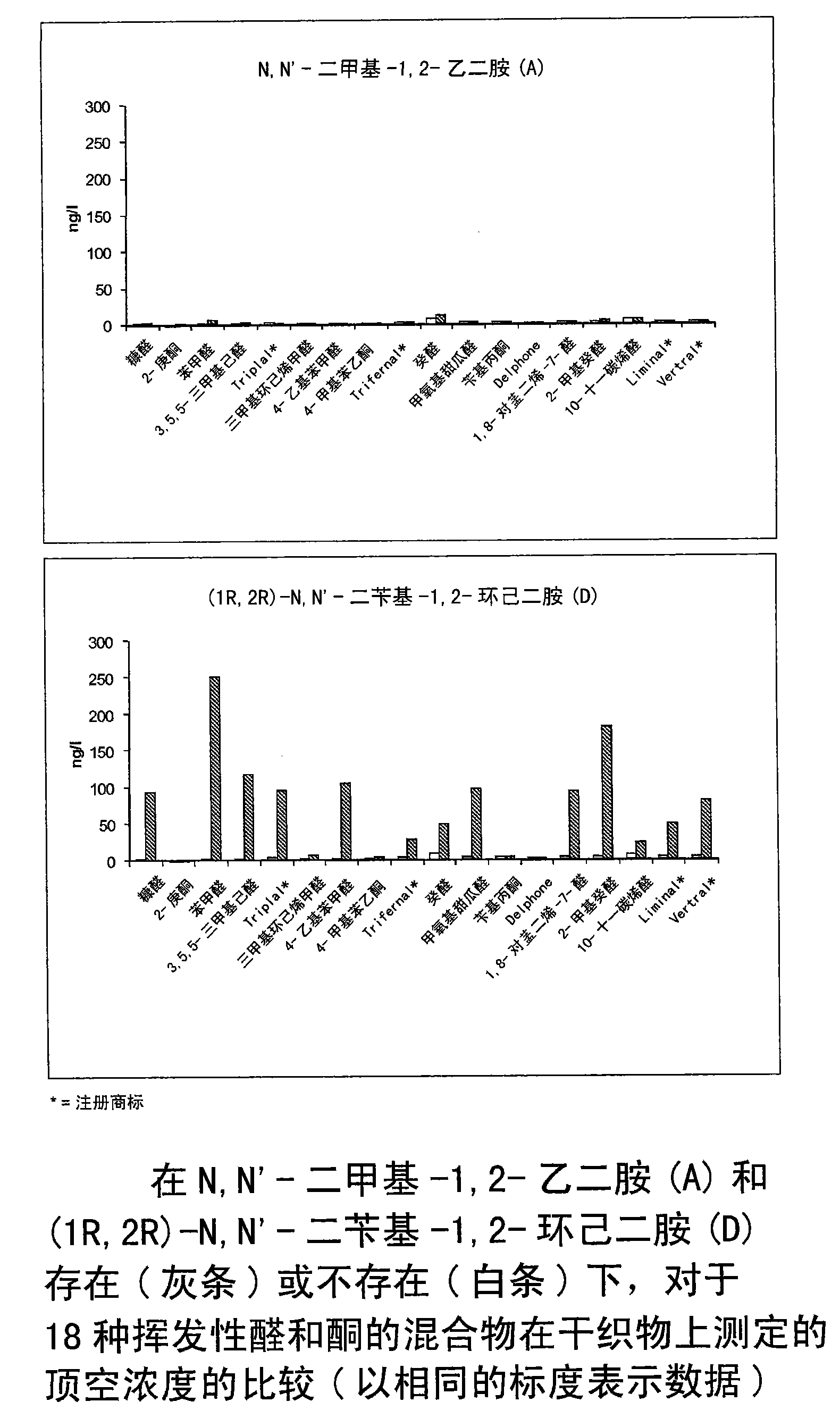
[0268] The perfuming performance over time of free aromatized aldehydes / ketones and mixtures according to the invention (i.e. free aromatized aldehydes / ketones and diamine derivatives as additives) was determined by the following test:
[0269] Weigh (1R,2R)-N,N'-dibenzyl-1,2-cyclohexanediamine (73.4 mg, 2.46 mmol) into a vial. Then add 1.80 g of the above fabric softener base, containing equimolar amounts (0.41 mmol) of 2-furfuraldehyde (furfural, 39.4 mg), (R)-3,7-dimethyl-6-octane in 10 ml of ethanol Enal (citronellal, 63.2mg), 3-phenylbutyraldehyde ( 60.8 mg), 2-pentyl-1-cyclopentanone (Delphone, 63.2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com