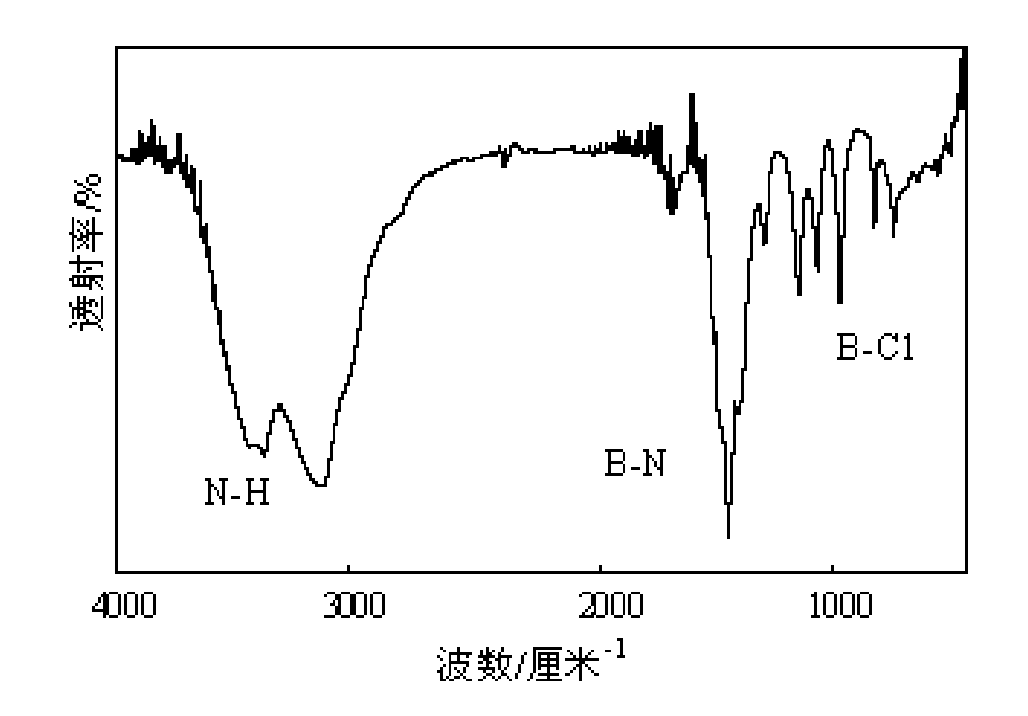
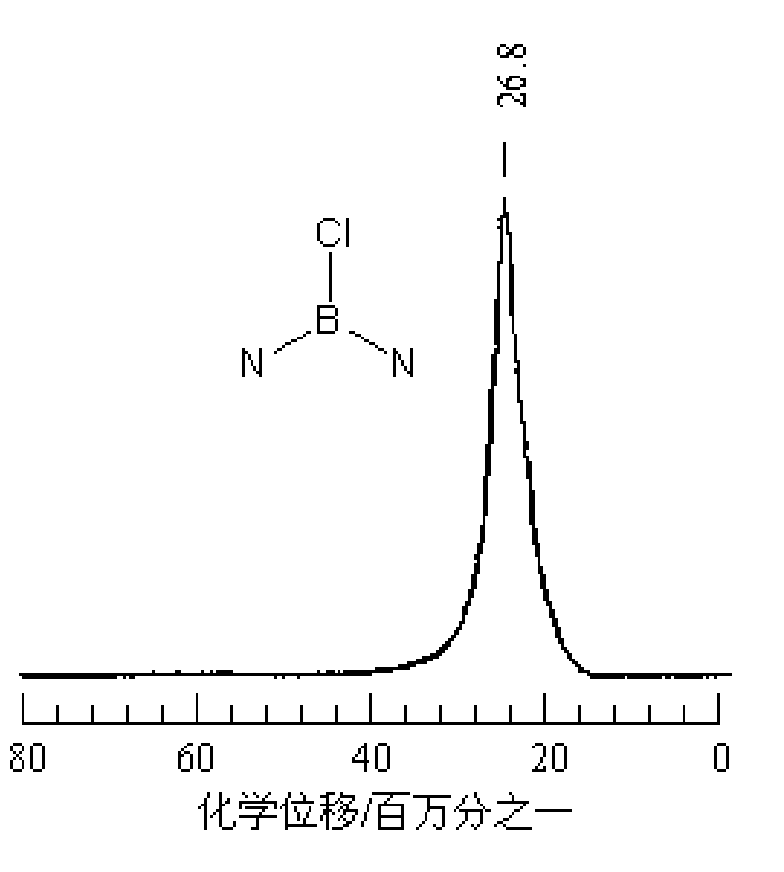
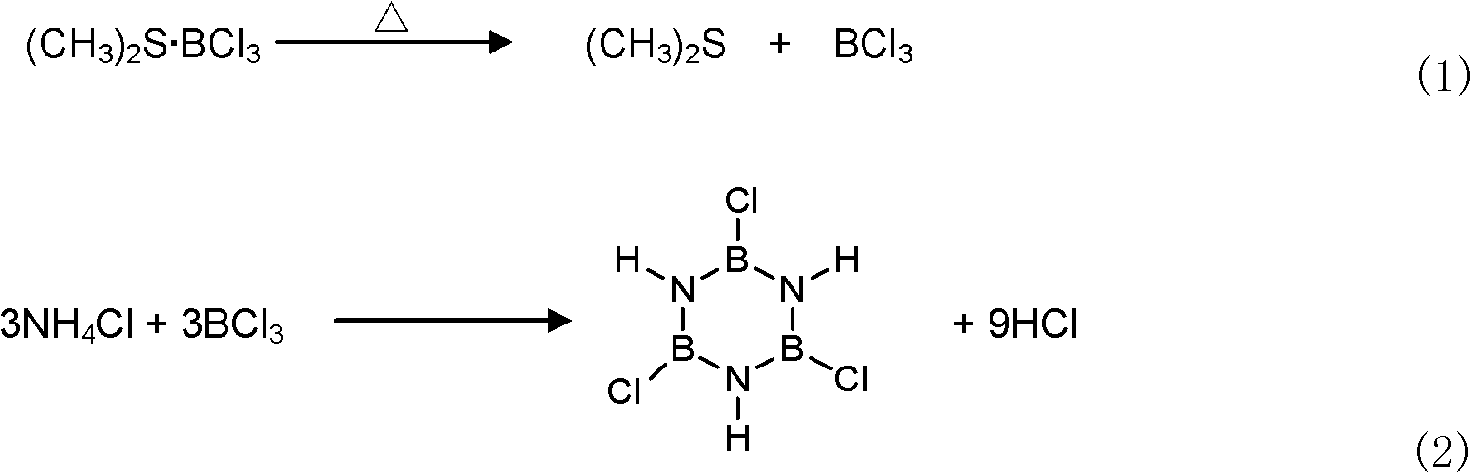
Synthetic method of B-trichloroborazine
A technology of trichloroborazine and a synthesis method, which is applied in the field of synthesis of trichloroborazane, can solve the problems of difficult boron trichloride feeding amount, difficult experimental operation, low product synthesis yield and the like, and achieves the The effect of improving the utilization rate of boron source, easy mass production, and improving synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) 95.7g dimethylsulfur boron trichloride ((CH 3 ) 2 S·BCl 3 ) and 20g ammonium chloride (NH 4 C1) The powder is placed in a 1000ml flask after being fully mixed directly, and 500ml of toluene is added and mixed uniformly;
[0019] (2) Add the above-mentioned dispersed turbid liquid into the reactor with magnetic stirring and low-temperature condensation reflux device, repeatedly vacuumize and fill dry nitrogen at least three times to get rid of the air and moisture in the reaction device, then slowly raise the temperature to 110°C, While vigorously stirring, condense and reflux at the outlet end at a low temperature of -20 to -10°C for 15 hours.
[0020] (3) cooling to room temperature, filtering, and recovering the organic solvent toluene through vacuum distillation to obtain 21.7 g of colorless needle-like crystal product with a yield of 94.8% and a boron source utilization rate of 66.3%.
[0021] The appearance of the product obtained in Example 1 is white need...
Embodiment 2
[0023] (1) 99.5g dimethylsulfur boron trichloride ((CH 3 ) 2 S·BCl 3 ) and 25g ammonium sulfate ((NH 4 ) 2 SO 4 ) powder is placed in a 1000ml flask after being fully mixed directly, and 500ml of chlorobenzene is added and mixed evenly;
[0024] (2) Add the above-mentioned dispersed turbid liquid into the reactor with ultrasonic dispersion stirring and low-temperature condensation reflux device, repeatedly vacuumize and fill with dry nitrogen at least three times to remove the air and moisture in the reaction device, and then slowly heat up to 130°C and violently The synthesis reaction was carried out under the condition of stirring, and at the same time, it was condensed and refluxed at the outlet end at a low temperature of -20~-10°C for 12 hours; after the reaction was completed, chlorobenzene was recovered by filtration and vacuum distillation to obtain 21.3 g of a colorless needle-shaped crystal product, with a yield of 91.7%, boron source utilization rate 62.6%.
Embodiment 3
[0026] (1) 95.0g dimethylsulfur boron trichloride ((CH 3 ) 2 S·BCl 3 ) and 20g ammonium chloride (NH 4 C1) The powder is placed in a 1000ml flask after being fully mixed directly, and 500ml xylene is added and mixed evenly;
[0027] (2) Add the above-mentioned dispersed turbid liquid into the reactor with high-speed mechanical stirring and low-temperature condensation reflux device, repeatedly vacuumize and fill with dry nitrogen at least three times to remove the air and moisture in the reaction device, and then slowly raise the temperature to 140°C, While vigorously stirring, condense and reflux at the outlet end at a low temperature of -20 to -10°C for 12 hours. After the reaction was completed, the xylene was recovered by filtration and vacuum distillation to obtain 21.4 g of a colorless needle crystal product with a yield of 93.3% and a boron source utilization rate of 65.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com