Dihydroxyoleic acid, preparation method and application thereof
A technology of bishydroxyoleic acid and bishydroxyl, applied in the preparation of carboxylates, preparation of organic compounds, preparations for skin care, etc., can solve problems such as differences, achieve high yield, mild reaction conditions, and improve immunity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Into a 50 mL round bottom flask was added 383 mg of SeO 2 , 10 mL of dichloromethane, stirred at 25°C for 1 hour, slowly added 1 g of conjugated linoleic acid (conjugated linoleic acid contains cis 9, trans 11-conjugated linoleic acid and trans 10, cis 12-conjugated Two isomers of linoleic acid, with a purity of 95%) were stirred and reacted for 48 hours. After the reaction, add water to wash, collect the dichloromethane layer, and recycle the dichloromethane to obtain 0.98 g of light yellow oil with a yield of 70.8% and a purity of 90% (W / W).
[0094] Analysis of the product: the light yellow oil obtained by the reaction is separated by semi-preparative normal phase high-performance liquid chromatography, and the separation conditions: the mobile phase is n-hexane: Virahol (volume ratio) is 98: 2; flow rate 8mL / min ; The detector is a differential refractive index detector; the sample is dissolved in n-hexane with a concentration of 100 mg / mL; the sample injection is ...
Embodiment 2
[0132] Add 200 mg of SeO to a 50 mL round bottom flask 2 , 5mL of dichloromethane, stirred at 60°C for 1 hour, slowly added 2g of conjugated linoleic acid with a purity of 95%, and continued to stir for 12 hours. After the reaction, add water to wash, collect the dichloromethane layer, and recover the dichloromethane to obtain 0.8 g of light yellow oil with a yield of 40% and a purity of 88% (W / W). The product was analyzed by normal phase high performance liquid chromatography.
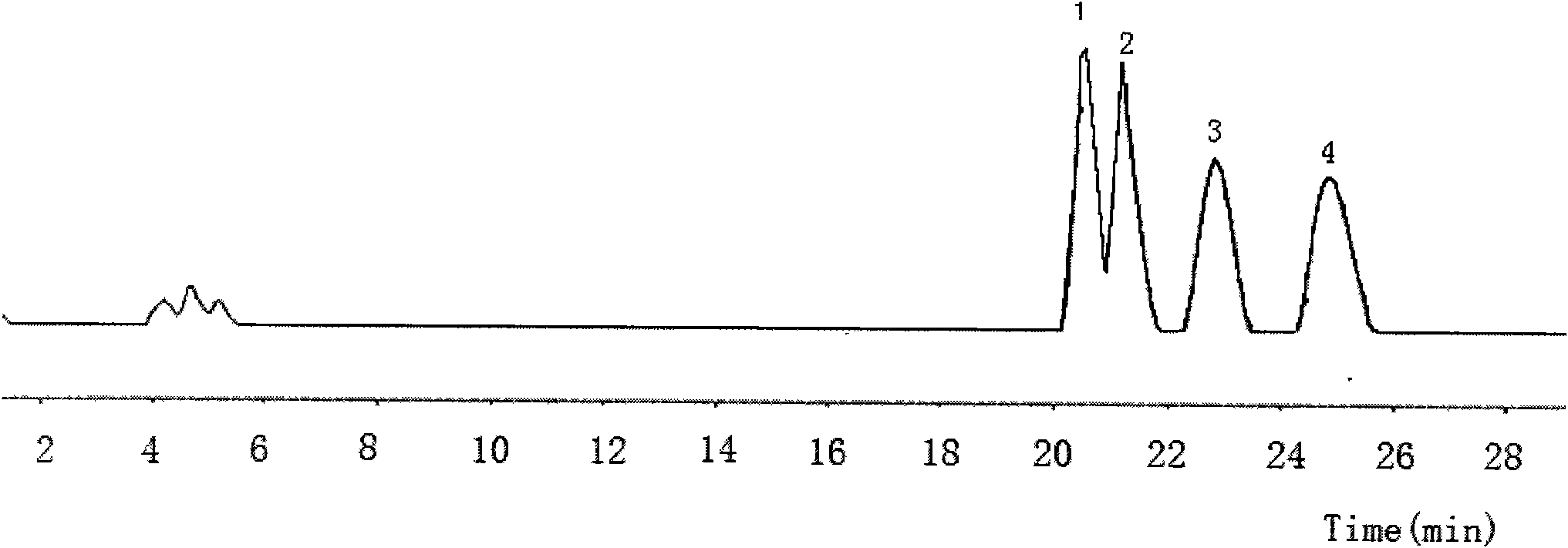
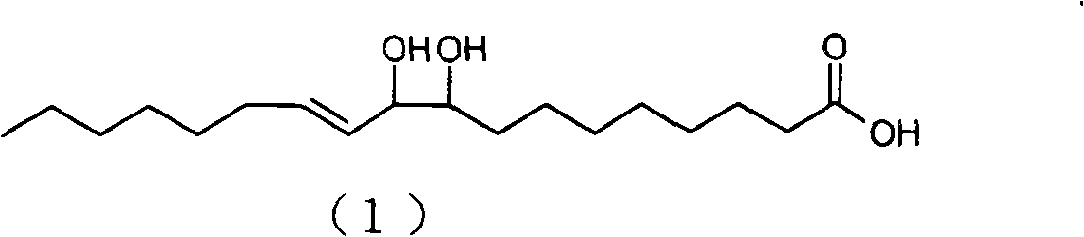
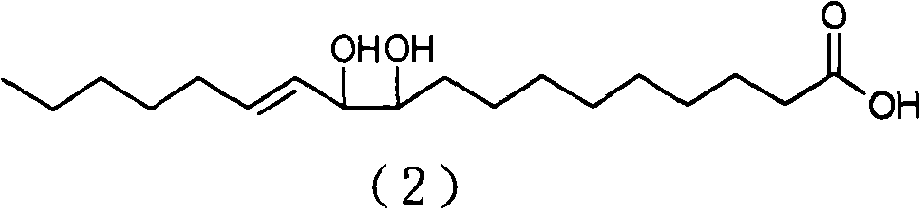
[0133] Analysis conditions: the mobile phase is n-hexane / isopropanol (volume ratio) 98 / 2; the flow rate is 2 mL / min; the detector is a differential refractive index detector; the sample concentration is 20 mg / mL; the sample injection is 10 μL. Under this separation condition, obtain four product peaks, retention time is respectively 20.4min (peak 1), 21.3min (peak 2), 22.9min (peak 3), 24.9min (peak 4). figure 1 , confirmed as compounds: 12,13-dihydroxy-10E-octadecenoic acid, 11,12-dihydroxy-9E-octa...
Embodiment 3
[0135] Into a 50 mL round bottom flask was added 3.83 g of SeO 2, 200mL of toluene, stirred at 25°C for 2 hours, slowly added 10g of conjugated linoleic acid with a purity of 90%, and continued to stir for 48 hours. After the reaction, add water to wash, collect the toluene layer, and recover the toluene to obtain 9 g of light yellow oil, with a yield of 65.1% and a purity of 84% (W / W). Product adopts normal phase high performance liquid chromatography to analyze, and analysis condition is with embodiment 2, obtains four product peaks, retention time is respectively 20.4min (peak 1), 21.3min (peak 2), 22.9min (peak 3), 24.9min min (peak 4), compared with the standard of four isomers, confirmed to be compounds: 12,13-dihydroxy-10E-octadecenoic acid, 11,12-dihydroxy-9E-octadecenoic acid , 10,11-dihydroxy-12E-octadecenoic acid, 9,10-dihydroxy-11E-octadecenoic acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com