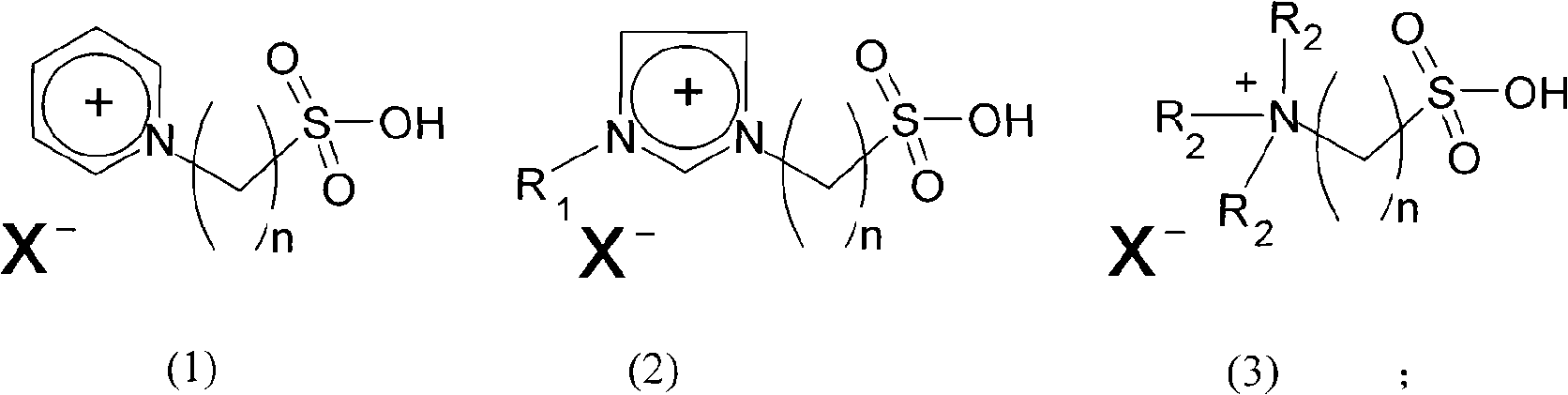
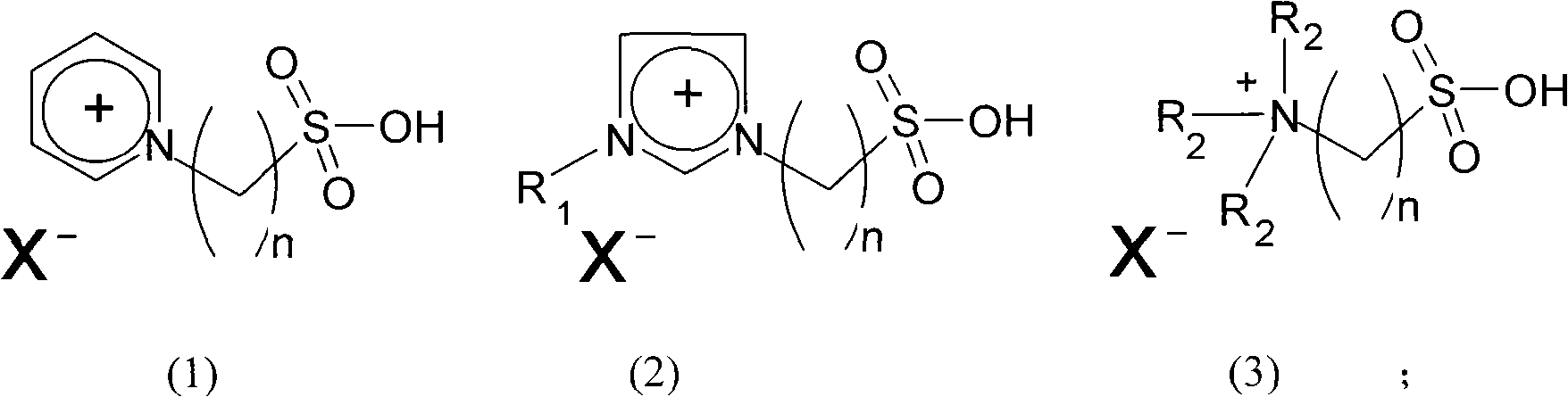
Method for preparing dimeric acid methyl ester
A technology of methyl dimer acid and acidity, which is applied in the field of preparation of methyl dimer acid, can solve the problems of high requirements on reaction equipment and operation, large amount of heterogeneous catalyst, harsh reaction conditions, etc., and achieves recycling performance. Good, easy separation and post-processing, short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: 1.0 g of 1-(4-sulfonic acid) butyl-3-methylimidazolium chloride zincate (wherein 1-(4-sulfonic acid) butyl-3-methylimidazolium chloride and chlorine The molar ratio of zinc chloride is 1: 3.0) and 30g of fatty acid methyl ester were reacted at 230°C for 6 hours under nitrogen protection, cooled to room temperature, added 30g of toluene to extract the polymerization product and unreacted raw materials, separated the upper layer extract, and Toluene and unreacted raw materials were removed under reduced pressure (5mmHg) at 240°C to obtain the polymerized product dimer acid methyl ester with a yield of 67%.
Embodiment 2
[0022] Example 2: 2.0 g of 1-(3-sulfonic acid) propyl-3-propylimidazolium chlorocuprate (wherein 1-(3-sulfonic acid) butyl-3-methylimidazolium chloride and chlorine The molar ratio of copper chloride is 1: 2.0) and 10 g of fatty acid methyl ester were reacted at 200° C. for 5 hours under nitrogen protection, cooled to room temperature, added 20 g of toluene to extract the polymerization product and unreacted raw materials, separated the upper layer extract, and Toluene and unreacted raw materials were removed under reduced pressure (5mmHg) at 240°C to obtain the polymerized product dimer acid methyl ester with a yield of 71%.
Embodiment 3
[0023] Example 3: 3.0 g of 1-(4-sulfonic acid) butyl-3-ethylimidazolium bromide cuprate (wherein 1-(4-sulfonic acid) butyl-3-ethylimidazolium bromide and bromine The molar ratio of copper chloride is 1: 1.1) and 12g of fatty acid methyl ester were reacted at 280°C for 4 hours under nitrogen protection, cooled to room temperature, added 25g of toluene to extract the polymerization product and unreacted raw materials, separated the upper layer extract, and Toluene and unreacted raw materials were removed under reduced pressure (5mmHg) at 240°C to obtain the polymerized product dimer acid methyl ester with a yield of 53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com