Method for extracting tripterine from celastrus orbiculatus root cortex
A technology of triptolide and eucalyptus, applied in the directions of steroids, organic chemistry, etc., can solve the problems of high extraction rate of raw materials and no mention of the preparation of triptolide monomers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
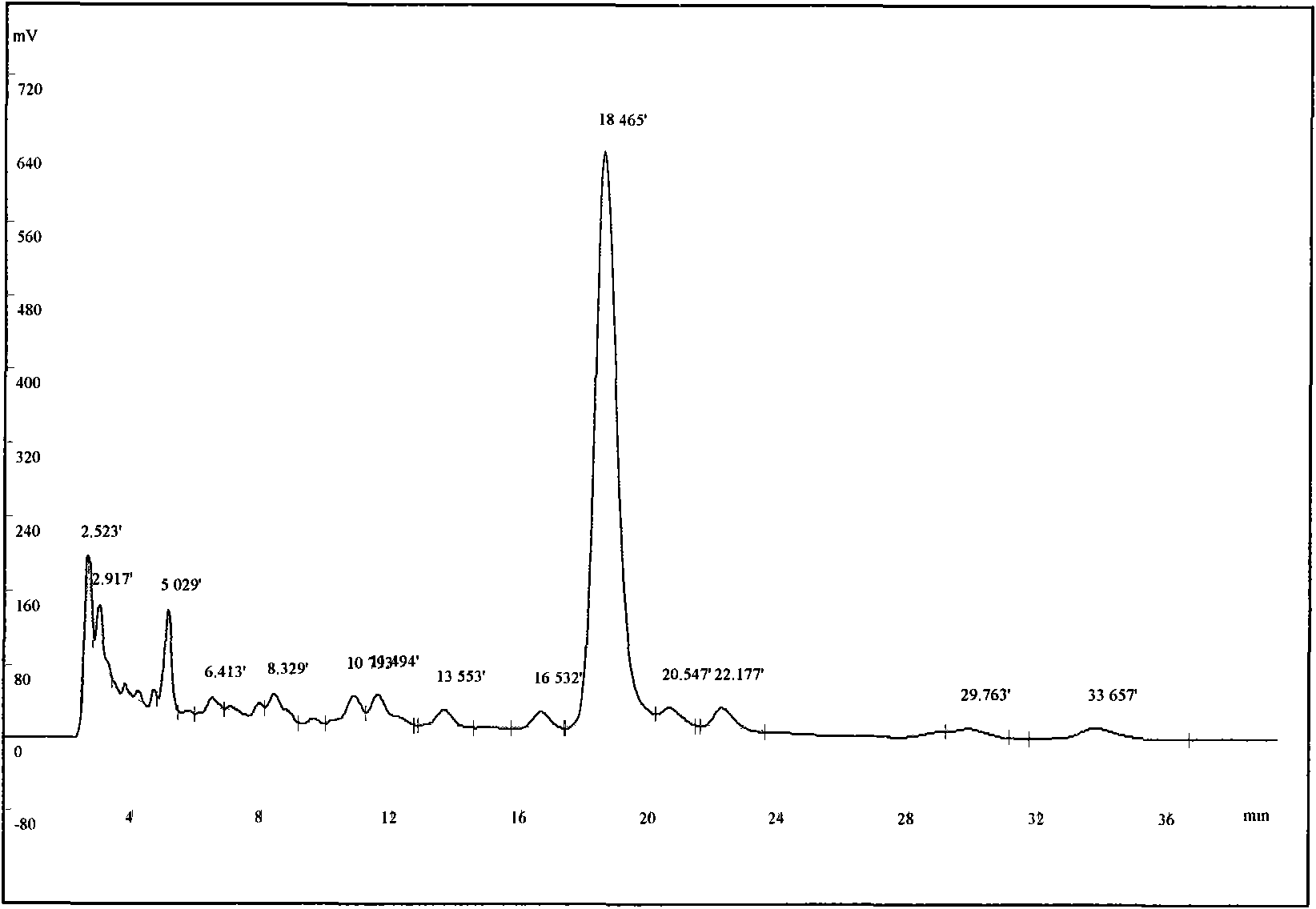
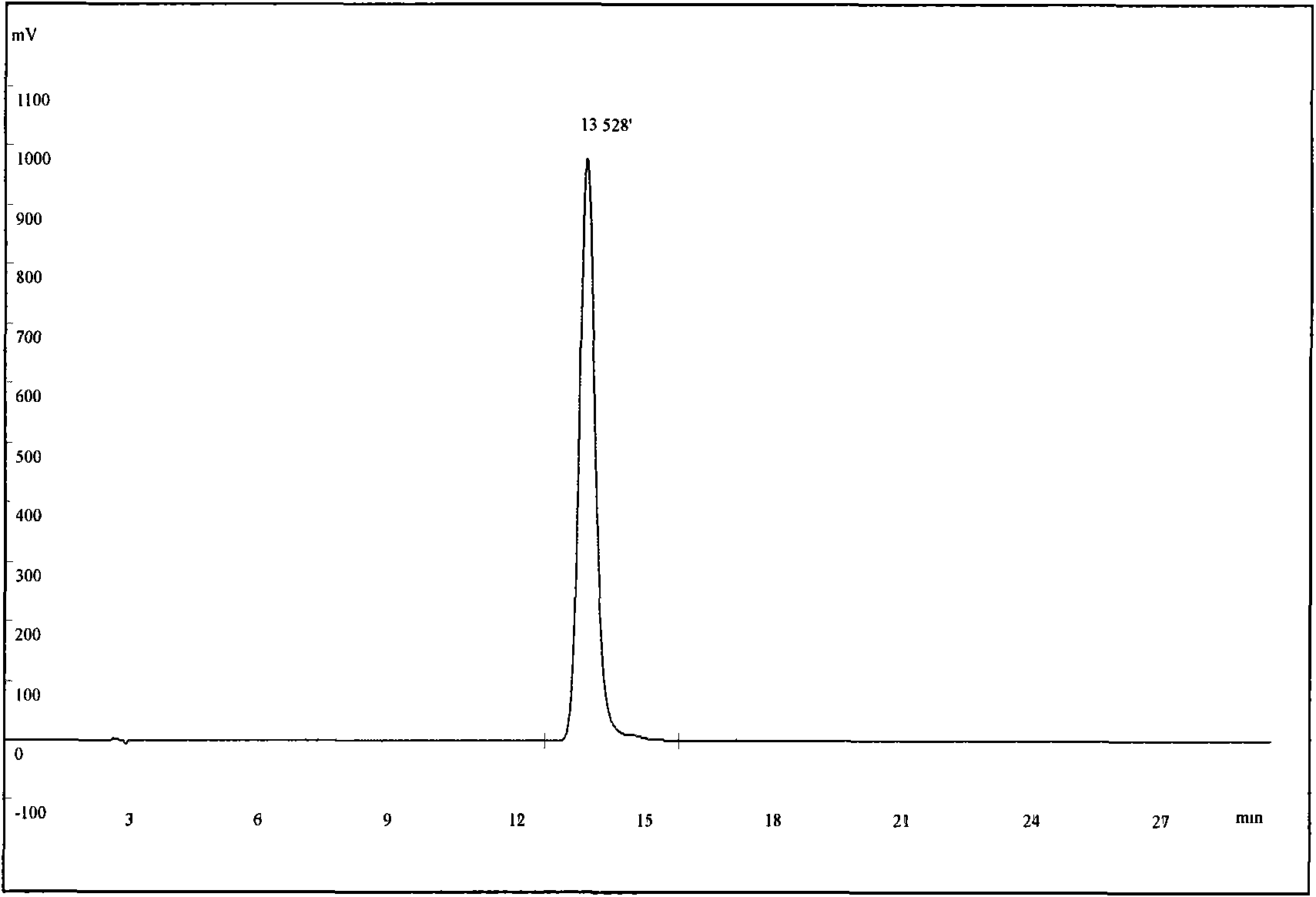
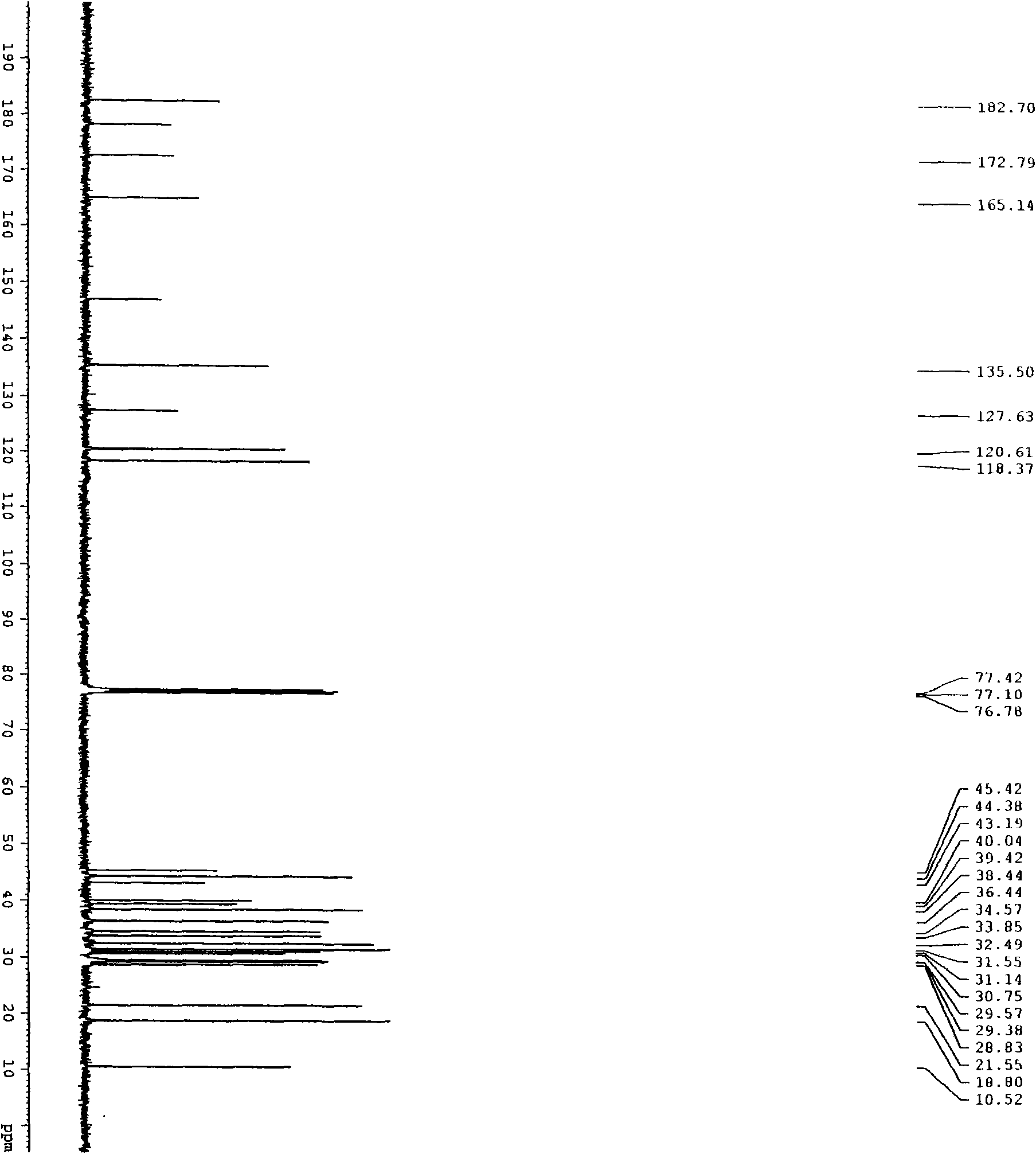
[0031] Select 300 g of the coarse powder of the root bark of S. chinensis with a particle size of 20 mesh, add 1,2-dichloroethane at a mass ratio of 1:5, heat and reflux for extraction for 4 hours, and reflux twice. After filtration, the combined filtrates were concentrated under reduced pressure to recover 1,2-dichloroethane to obtain 0.39 g of dark red extract (primary extract) with a red pigment content of 9.2%. Add 50vt% methanol solution to dissolve, a white precipitate precipitates out of the solution, let it stand still, take the supernatant to concentrate and repeat the treatment 2-3 times to obtain the crude extract of tripteryglide, the content of tripteryne is 51.3%. The crude extract was dissolved in methanol and the stationary phase was reversed phase C 18 Separation is carried out on the preparative chromatography, the eluent is 83vt% methanol aqueous solution, the pH is adjusted to 3 with acetic acid, the tripterylide fractions are collected according to the pre...
Embodiment 2
[0033] Select 300 g of the coarse powder of the root bark of S. chinensis with a particle size of 20 mesh, add metatrichloroethane at a mass ratio of 1:7, heat and reflux for extraction for 5 hours, and reflux twice. After filtering, the combined filtrates were concentrated under reduced pressure to recover metatrichloroethane to obtain 0.41 g of dark red extract (initial extract) with tripterine content of 10.3%. Add 60vt% ethanol solution to fully dissolve, white precipitate precipitates out in the solution, take the filtrate after filtration, repeat the same treatment 2-3 times again after concentrating, and obtain tripteryglide crude extract, tripteryne content 48.2%. The crude extract was dissolved in methanol and the stationary phase was reversed phase C 18 Separation is carried out on the preparative chromatography, the eluent is 85vt% methanol solution, the pH is adjusted to 3 with acetic acid, the tripterylide fraction is collected according to the preparative chromat...
Embodiment 3
[0035]Select 300 g of the coarse powder of the root bark of S. chinensis with a particle size of 20 mesh, add chloroform at a mass ratio of 1:10, heat and reflux for extraction for 5 hours, and reflux twice. After filtering, the combined filtrates were concentrated under reduced pressure to recover chloroform to obtain 0.47 g of dark red extract (initial extract) with tripterine content of 10.1%. Add 60vt% methanol solution, the solution is turbid, let it stand, filter and concentrate the filtrate and repeat the same treatment 2-3 times again to obtain the tripterylide crude extract with a tripterylide content of 53.1%. The crude extract was dissolved in methanol and mixed with silica gel to separate the stationary phase into a normal-phase silica gel atmospheric pressure column. The eluent was chloroform / methanol (60:40) isocratic elution, followed by TLC monitoring, and the tripterine fraction was collected and concentrated. After evaporating to dryness, the essence-producin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com