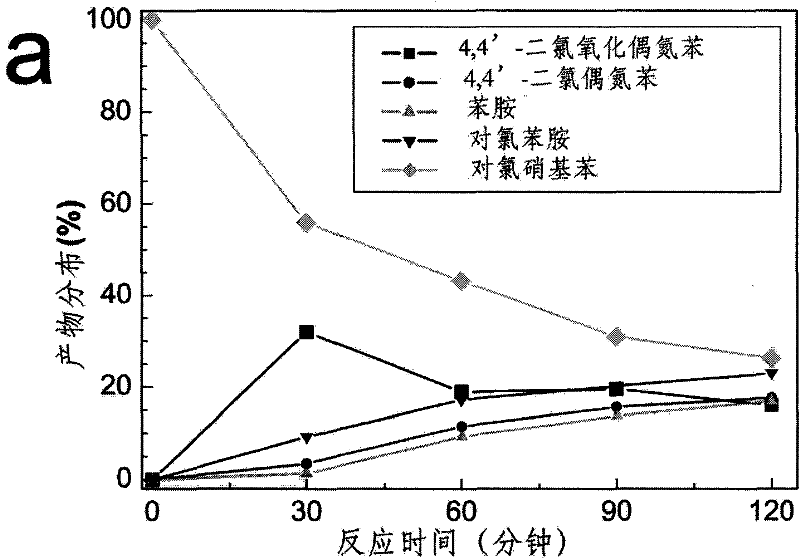
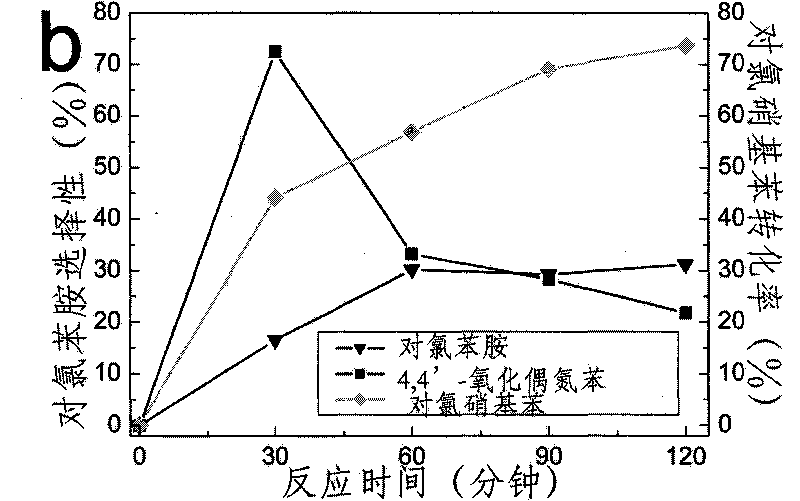
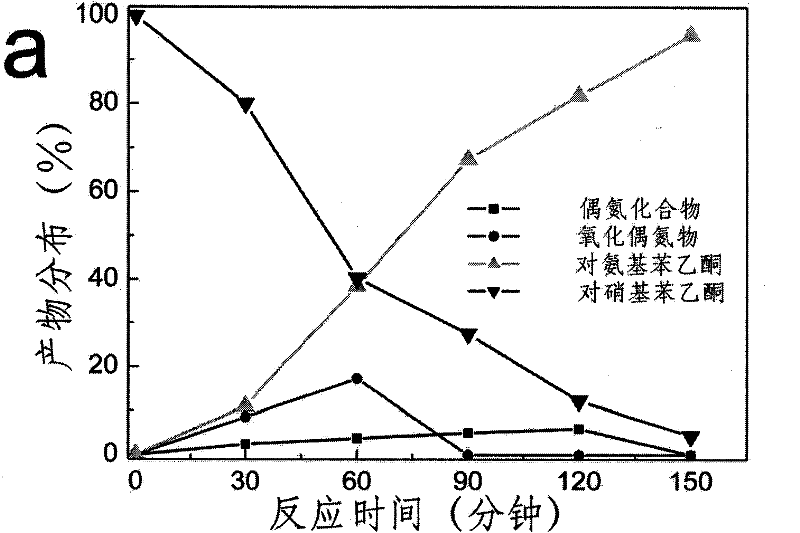
Application of fullerenes as catalysts in the catalytic hydrogenation of nitro groups in nitroaromatic compounds under light irradiation
A nitroaromatic and catalytic hydrogenation technology, which is applied in the preparation of amino compounds, organic compounds, physical/chemical process catalysts, etc., can solve the problems of high cost, waste of resources, pollution of the environment, etc., and achieve high selectivity and low Ecological hazard and environmental hazard, highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The preparation method of each substance is as follows:
[0022] Monovalent C 60 Preparation of negative ions: in 25mL tetrahydrofuran, add 0.036g C 60 , 0.130g Ni-Al alloy powder, 0.400g sodium hydroxide, under stirring, pass into nitrogen bubble to remove dissolved air, add freshly distilled distilled water 5.0mL after 30 minutes to initiate the reaction, after 60 minutes, stop stirring, stand still 30 minutes, the system is divided into two layers, monovalent C 60 Negative ions are dissolved in tetrahydrofuran, and the upper layer of tetrahydrofuran C is taken out 60 Negative ion solution, spare.
[0023] Monovalent C 70 Preparation of negative ions: in 25mL tetrahydrofuran, add 0.036g C 70 , 0.130g Ni-Al alloy powder, 0.400g sodium hydroxide, under stirring, pass into nitrogen bubble to remove dissolved air, add freshly distilled distilled water 5.0mL after 30 minutes to initiate the reaction, after 60 minutes, stop stirring, stand still 30 minutes, the system...
specific Embodiment 1
[0024] Photo-excited catalytic reaction test: The photo-excited catalytic reaction was carried out under the irradiation of a 300W high-pressure Hg lamp, and 0.036g of catalyst C was added to the columnar quartz reactor 60 , 1.0g of nitrobenzene reaction substrate and 250ml of tetrahydrofuran solvent, bubbling hydrogen for 30 minutes, removing dissolved air, turning on the light source, and bubbling hydrogen under magnetic stirring, the temperature of the whole system is maintained at 25°C by circulating water cooling, and the reaction After 1.0 hour, the stirring and hydrogen bubbling were stopped, and a sample of the reaction solution was taken out for GC-MS analysis. The catalyst was recovered and characterized by centrifugation. The conversion rate of nitrobenzene was 82.6%, and the selectivity of aniline was 78.9%.
specific Embodiment 2
[0025] Photo-excited catalytic reaction test: The photo-excited catalytic reaction was carried out under the irradiation of a 300W high-pressure Hg lamp, and 0.036g of catalyst C was added to the columnar quartz reactor 60 , 1.0g nitrobenzene and 250ml tetrahydrofuran solvent, bubbling hydrogen for 30 minutes, removing dissolved air, turning on the light source, and bubbling hydrogen under magnetic stirring. , stop stirring and hydrogen bubbling, take out a sample of the reaction solution for GC-MS analysis, and the catalyst is recovered and characterized by centrifugation. The conversion rate of nitrobenzene is 100%, and the selectivity of aniline is 92.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com