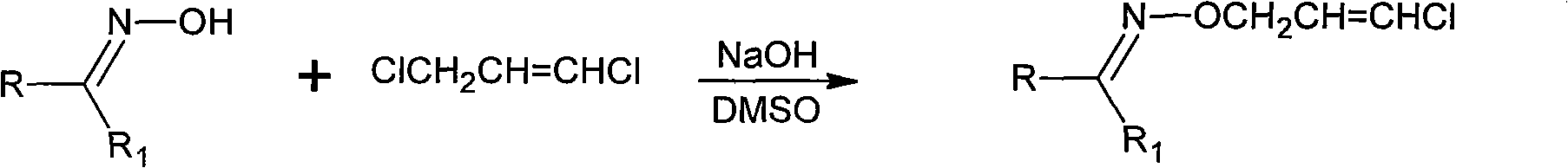
Method for synthesizing o-trans-(3-Cl-2-propenyl) hydroxylamine hydrochloride
A hydroxylamine hydrochloride and propylene-based technology, which is applied in the field of compound synthesis, can solve the problems of difficult industrialization and high requirements for production equipment, and achieve the effects of industrialized production, saving raw materials, and safe production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Add 174gDMSO, 26.4g (0.66mol) NaOH to a 250mL flask equipped with an electric stirrer, a thermometer, and a condenser at room temperature, stir and dissolve at room temperature, and then 43.5g (0.50mol) butanone oxime;
[0040] (2) Add 63.0g (0.55mol) trans-1,3-dichloropropene (96.0%) dropwise at 20-25°C;
[0041] (3) After the dropwise addition, react at a temperature of 20-30°C for 1 hour:
[0042](4) Then cool to room temperature, extract with cyclohexane 65g×4 (that is, the amount of each extraction is 65g, and extract 4 times in total), combine the upper layer extracts, and evaporate the cyclohexane in the extraction layer to separate O-(3 -Chloro-2-propenyl) ketone oxime ether obtains gross weight and is 338.2g;
[0043] (5) Then add 78.2g of 35% HCl (0.75mol) to the O-(3-chloro-2-propenyl)ketoxime ether obtained in step (4);
[0044] (6) Prepare a glass vacuum rectification column with an internal diameter of 20 mm and a height of 1600 mm with spring glass ...
Embodiment 2
[0046] Butanone oxime is changed into 36.5g (0.50mol) acetone oxime in step (1), other steps are with embodiment 1, the result is reclaiming O-(3-chloro-2-propenyl) acetone oxime ether 39.8g, O-reverse Formula-(3-chloro-2-propenyl)hydroxylamine hydrochloride 27.3g, yield 82.4%, product molar fraction is 99.0%.
Embodiment 3
[0048] In the step (1), the dimethyl sulfoxide consumption is changed to 220g, and other steps are with embodiment 1, the result is reclaiming O-(3-chloro-2-propenyl) butanone oxime ether 43.4g, O-trans-( 27.7 g of 3-chloro-2-propenyl) hydroxylamine hydrochloride, the yield is 83.1%, and the product molar fraction is 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com