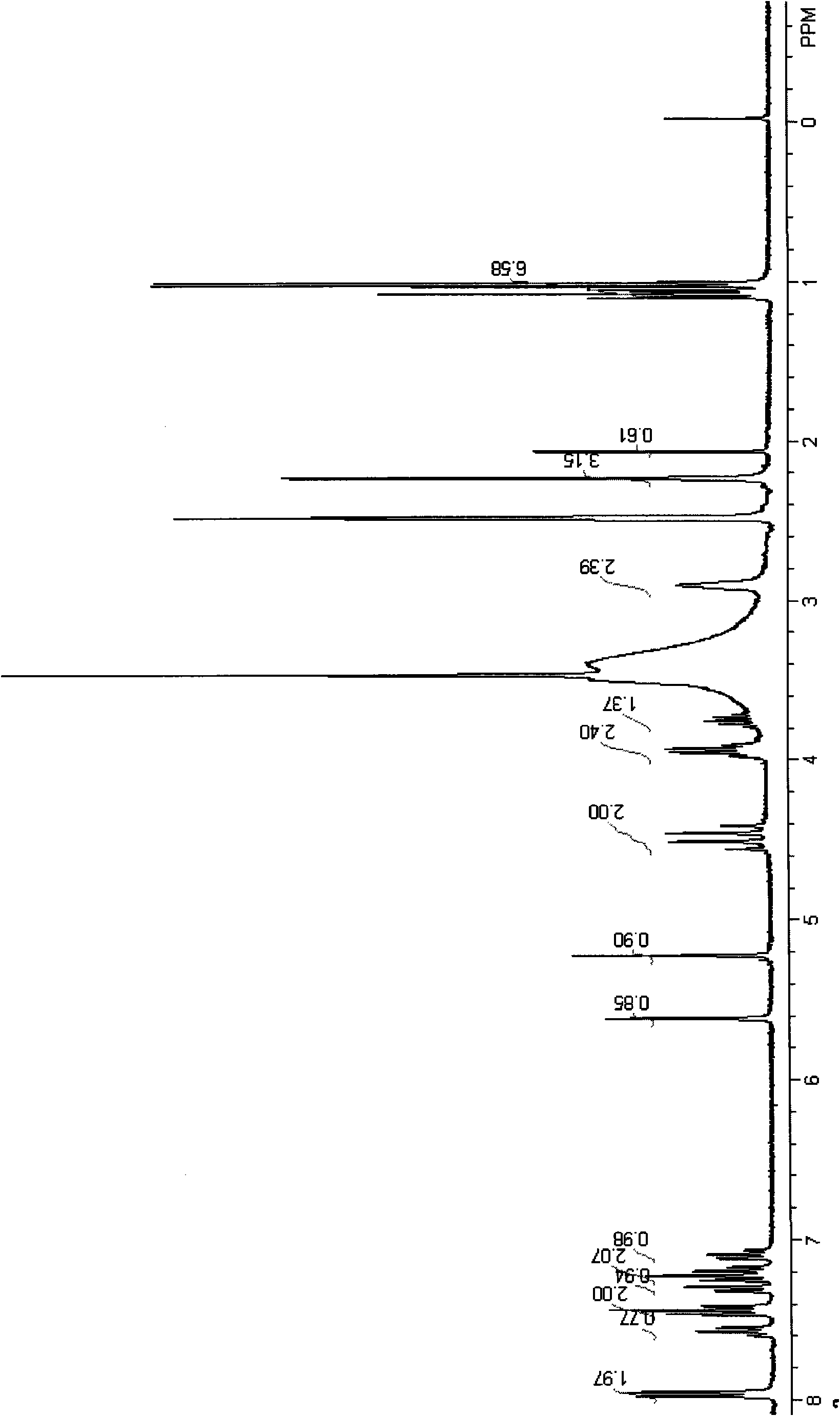
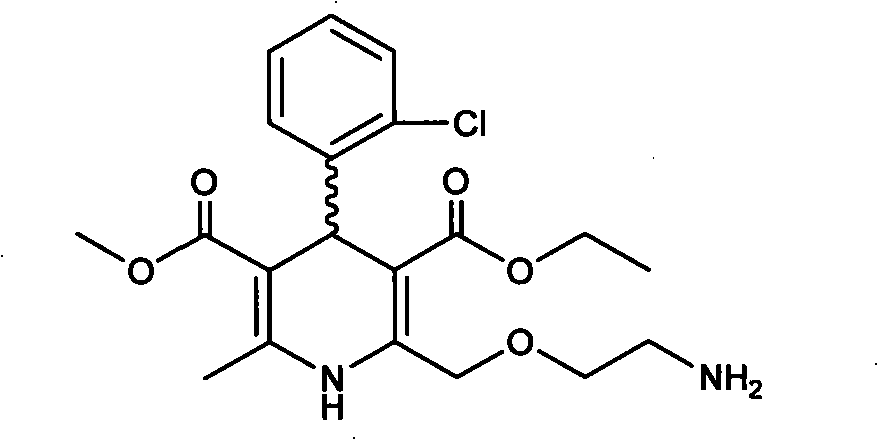
Method for preparing optically active amlodipine tosilate
A technology of p-toluenesulfonate and p-toluenesulfonic acid, which is applied in the field of preparation of amlodipine salt, can solve problems such as pollution, toxicity of deuterated reagents, and difficulty in recycling, so as to reduce production costs, prevent environmental pollution, and solvent toxicity small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0026] Example 1: Preparation of S-(-)-amlodipine-semi-di-p-methylbenzoyl-D-tartrate from (R,S)-amlodipine
[0027] 0.13g O, O'-di-p-methylbenzoyl-D-tartaric acid (0.25 molar equivalent) and 0.50g amlodipine were dissolved in 10mL of isopropanol; Stir for 10 to 15 minutes, add O, O'-di-p-toluyl-D-tartaric acid isopropanol solution to the above solution under stirring, continue stirring for 10 minutes, stop heating, and continue stirring for 2 Hour, no white precipitate is generated, and the solution is clear and transparent. Add 0.02g O, O'-di-p-toluyl-D-amlodipine tartrate salt as a seed crystal, and a white precipitate is produced rapidly; continue to stir for 3 hours, Filtered, dried, weighed 0.37g, vacuum dried for 3 hours and weighed to obtain a gross weight of 0.36g (the actual weight was 0.34g, that is, the gross weight minus the mass of the 0.02g seed crystal) and the yield was 85% (theoretical yield was 0.4g). m.p.: 123-127°C. Found values: C: 59.81%, H: 5.86%, N: 4...
example 2
[0028] Example 2: Preparation of S-(-)-amlodipine p-toluenesulfonate from S-(-)-amlodipine-semi-di-p-toluene-D-tartrate
[0029] The S-(-)-amlodipine-semi-di-p-toluoyl-D-tartrate 0.34g solid prepared in Example 1 was placed in a 100mL conical flask, and 10mL of dichloromethane and 10mL of Sodium hydroxide solution (2mol / L). Stir at room temperature for 1.5 hours. Transfer the mixed liquid to a separatory funnel, let it stand, separate the layers, wash the aqueous layer twice with dichloromethane, 10mL each time, combine the organic layer, wash the organic layer twice with water, 10mL each time, and then saturated with 10mL Wash once with salt water, the organic layer becomes clear and transparent, separate the liquid into a dry Erlenmeyer flask; add anhydrous sodium sulfate and dry for 30 minutes; filter, wash anhydrous sodium sulfate with dichloromethane; spin dry dichloromethane to obtain a yellow viscous, then add 0.2g p-toluenesulfonic acid and 20mL water, a white solid wi...
example 3
[0030] Example 3: Preparation of R-(+)-amlodipine p-toluenesulfonate
[0031] Spin the mother liquor in Example 1 to dryness, add 10mL of dichloromethane and 10mL of sodium hydroxide solution (2mol / L); stir at room temperature for 1.5 hours; transfer the mixed liquid to a separatory funnel, let it stand, separate liquid, water layer was washed twice with dichloromethane, 10 mL each time, the organic layers were combined, the organic layer was washed twice with water, 10 mL each time, and then washed once with 10 mL saturated brine, the organic layer became clear and transparent, and the liquid was separated to dryness. Add anhydrous sodium sulfate to dry for 30 minutes, filter, wash anhydrous sodium sulfate with dichloromethane; spin dry dichloromethane to obtain a yellow sticky substance; add 0.2g p-toluenesulfonic acid and 20mL water, After 10 minutes, a white solid will precipitate out, N 2 Heated under protection until the solids were completely dissolved, then cooled at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com