Preparation method of tripterygium glycosides solid lipid nanoparticle
A technology of lipid nanoparticles and tripterygium glycosides, which can be applied to medical preparations with no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve problems such as lack of pertinence, application limitations, and simple methods. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of tripterygium glycosides solid lipid nanoparticles (1 prescription amount)
[0031] Tripterygium glycosides 60mg
[0032] Glyceryl monostearate 360mg
[0033] Soy Lecithin 360mg
[0034] Poloxamer 60mg
[0035] Preparation Process:
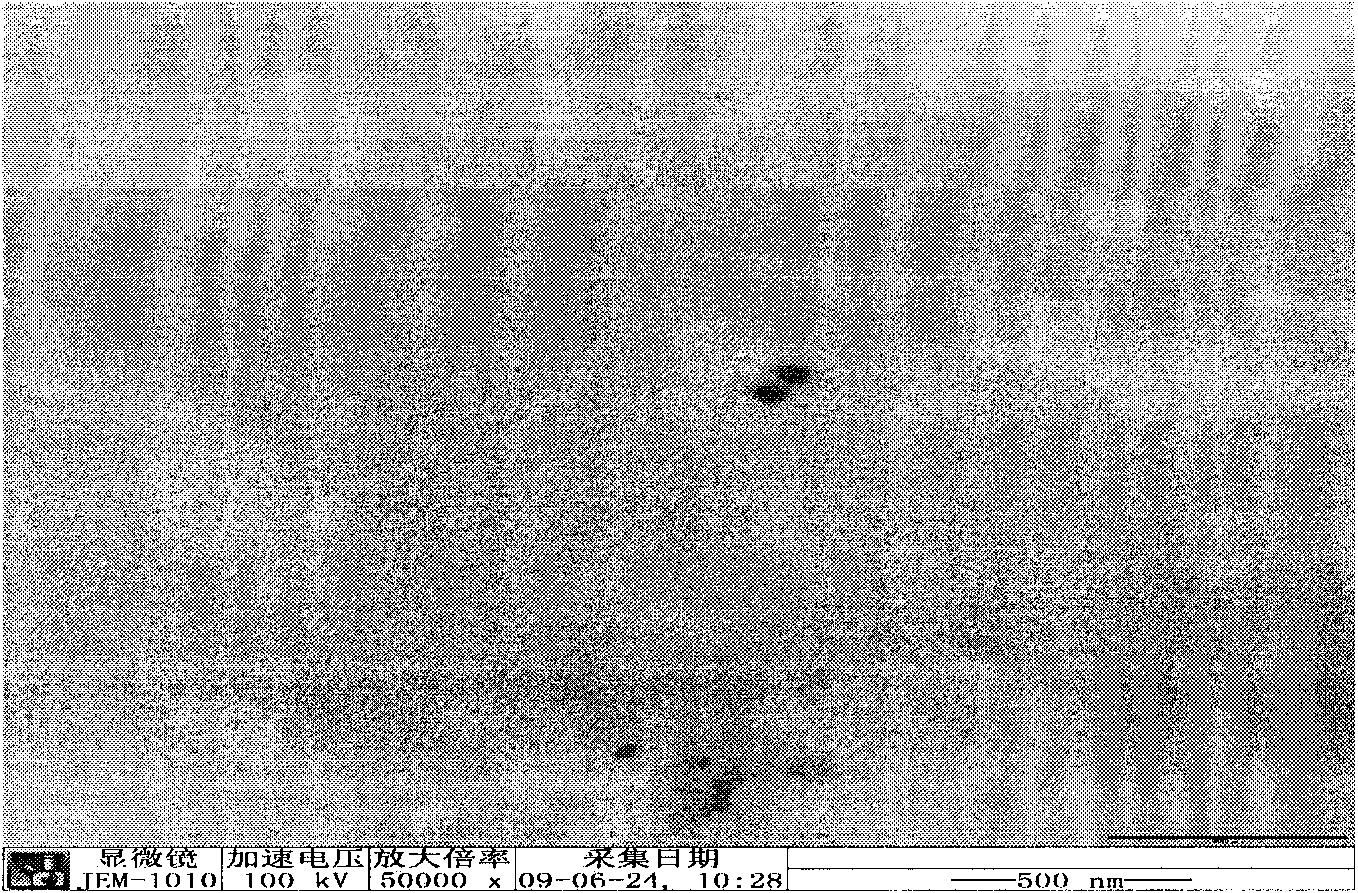
[0036] Add tripterygium glycosides and glyceryl monostearate to 8ml of acetone, heat and dissolve at 80°C; add soybean lecithin to 1ml of ethanol, and ultrasonically dissolve for 10min; mix the above two solutions as the organic phase; take another poloxamer solution Use 20ml of water as the water phase, inject the organic phase into the water phase at 80°C with a needle under stirring conditions, keep stirring for 4 hours, evaporate the organic solvent, quickly disperse the suspension into 30ml of water at 0-2°C, and solidify at low temperature for 2 hours. 0.45um microporous membrane filtration, that is. The average particle size of the obtained nanoparticles is: 204.5nm, the potential: -6.8mv
Embodiment 2
[0037] Example 2 Preparation of tripterygium glycosides solid lipid nanoparticles (1 prescription amount)
[0038] Tripterygium glycosides 60mg
[0039] Stearic acid 300mg
[0040]Soy Lecithin 300mg
[0041] Poloxamer 60mg
[0042] Preparation Process:
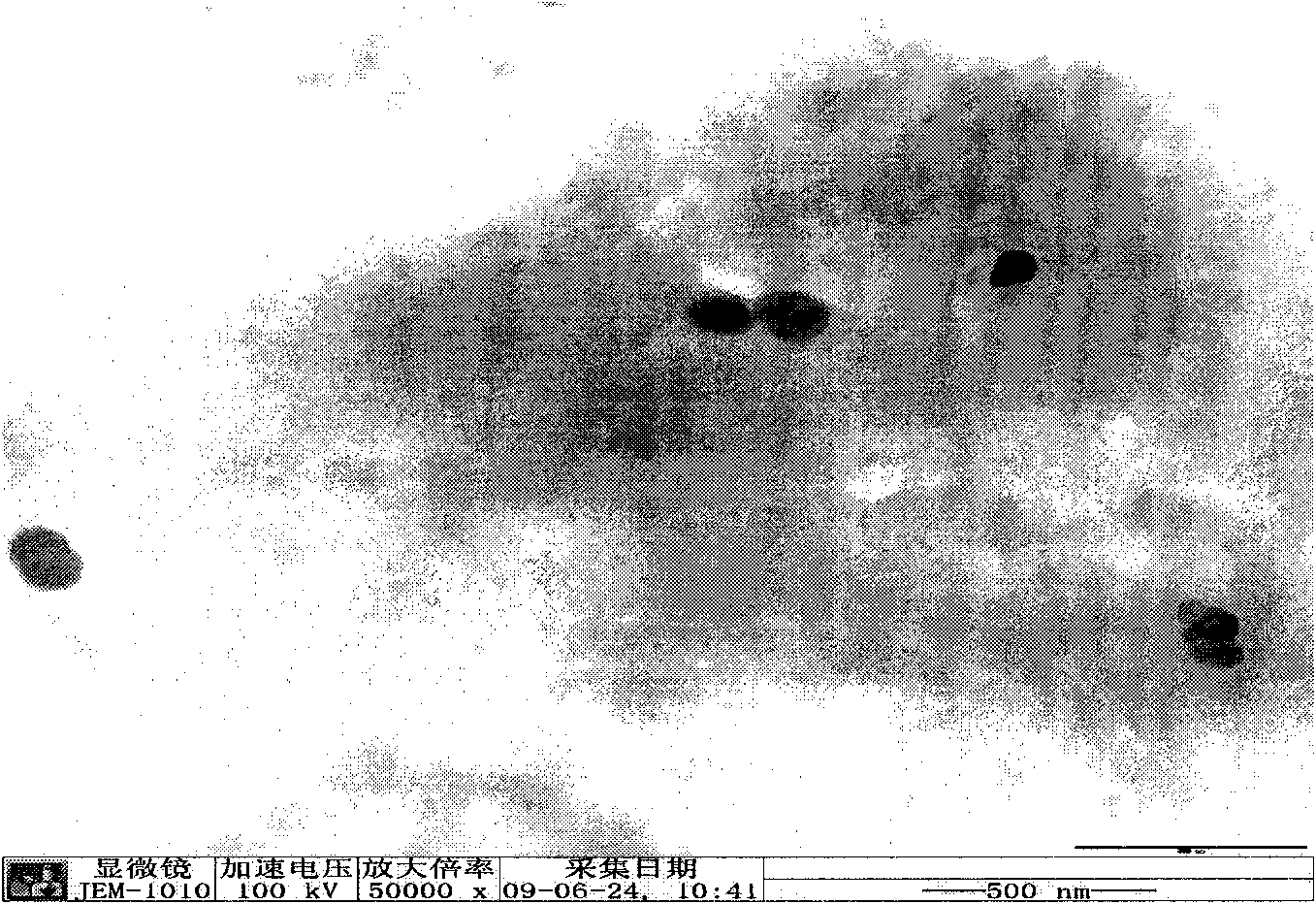
[0043] Dissolve tripterygium glycosides, stearic acid, and soybean lecithin in absolute ethanol; remove the organic solvent by rotary evaporation under reduced pressure to form a layer of lipid film, dry under reduced pressure overnight, then add the poloxamer aqueous solution, slowly Inject into the above-mentioned lipid film, sonicate for 1 hour, and filter through a 0.45um microporous membrane to obtain the product. The average particle size of the obtained nanoparticles is: 73.8nm Potential: -14.7mv
Embodiment 3
[0044] Example 3 Preparation of tripterygium glycosides solid lipid nanoparticles (1 prescription amount)
[0045] Tripterygium glycosides 60mg
[0046] Glyceryl monostearate 300mg
[0047] Glyceryl Caprylate 200ul
[0048] Soy Lecithin 60mg
[0049] Poloxamer 60mg
[0050] Preparation Process:
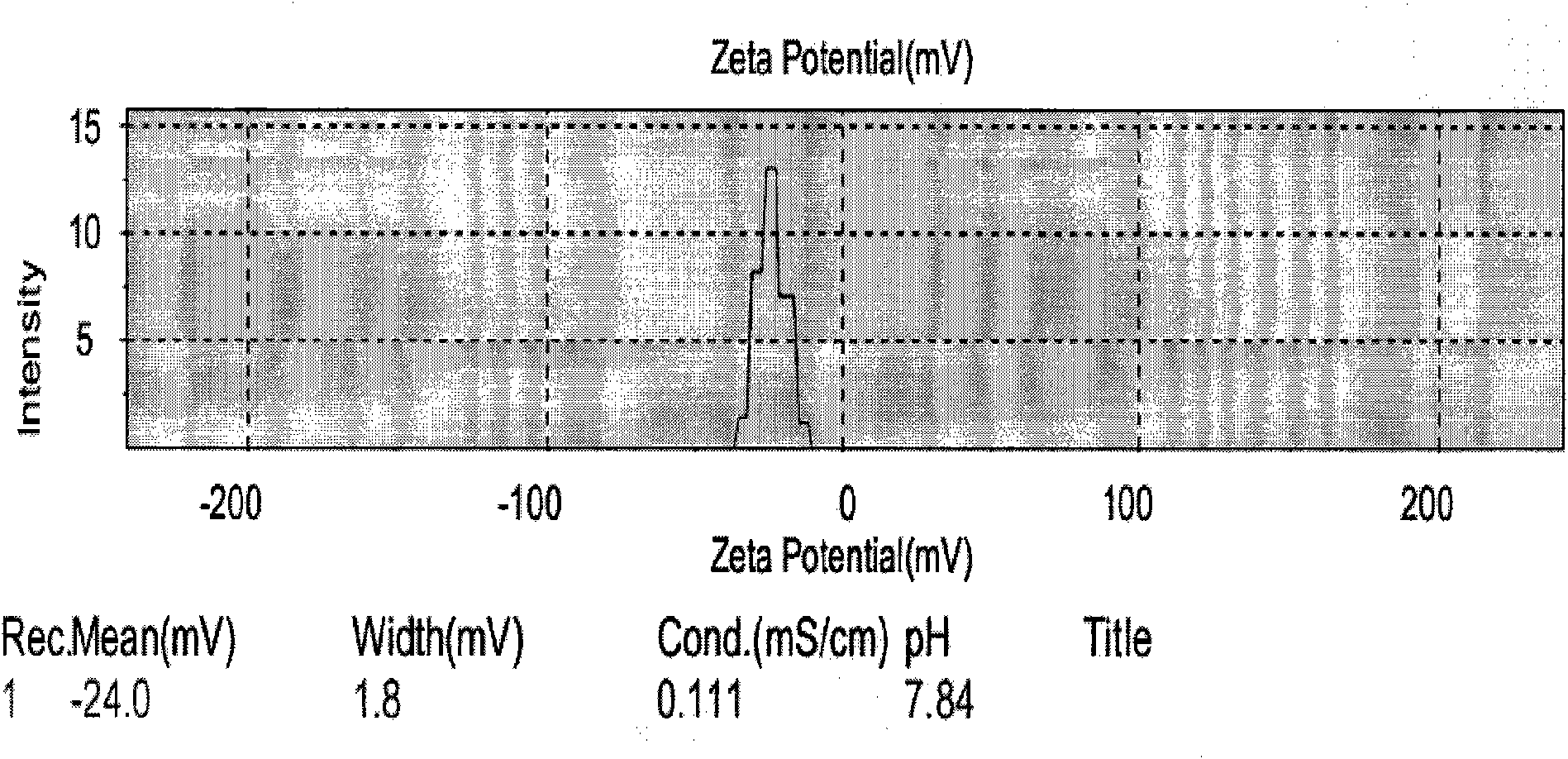
[0051] Add tripterygium glycosides to 80°C glyceryl monostearate, soybean lecithin, and glyceryl caprylate mixed lipid liquid; then disperse the hot lipid phase into poloxamer aqueous solution to obtain the initial dispersion, and homogenize under high pressure for 5 ~ 6 cycles, cooling and crystallization at 0 ~ 4 ° C to obtain. The average particle size of the obtained nanoparticles is: 182.7nm Potential: -27.6mv
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com