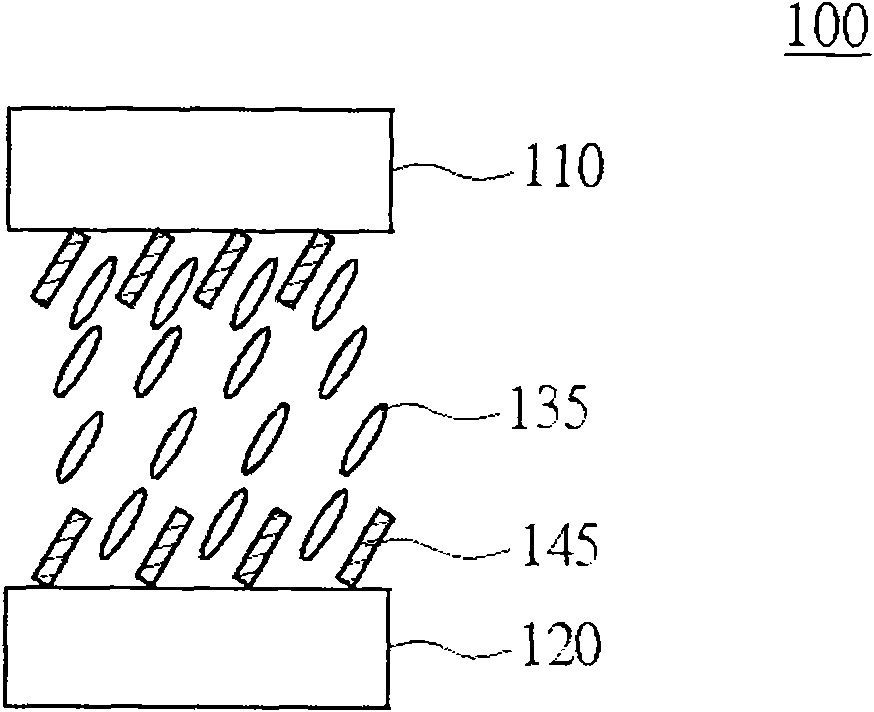
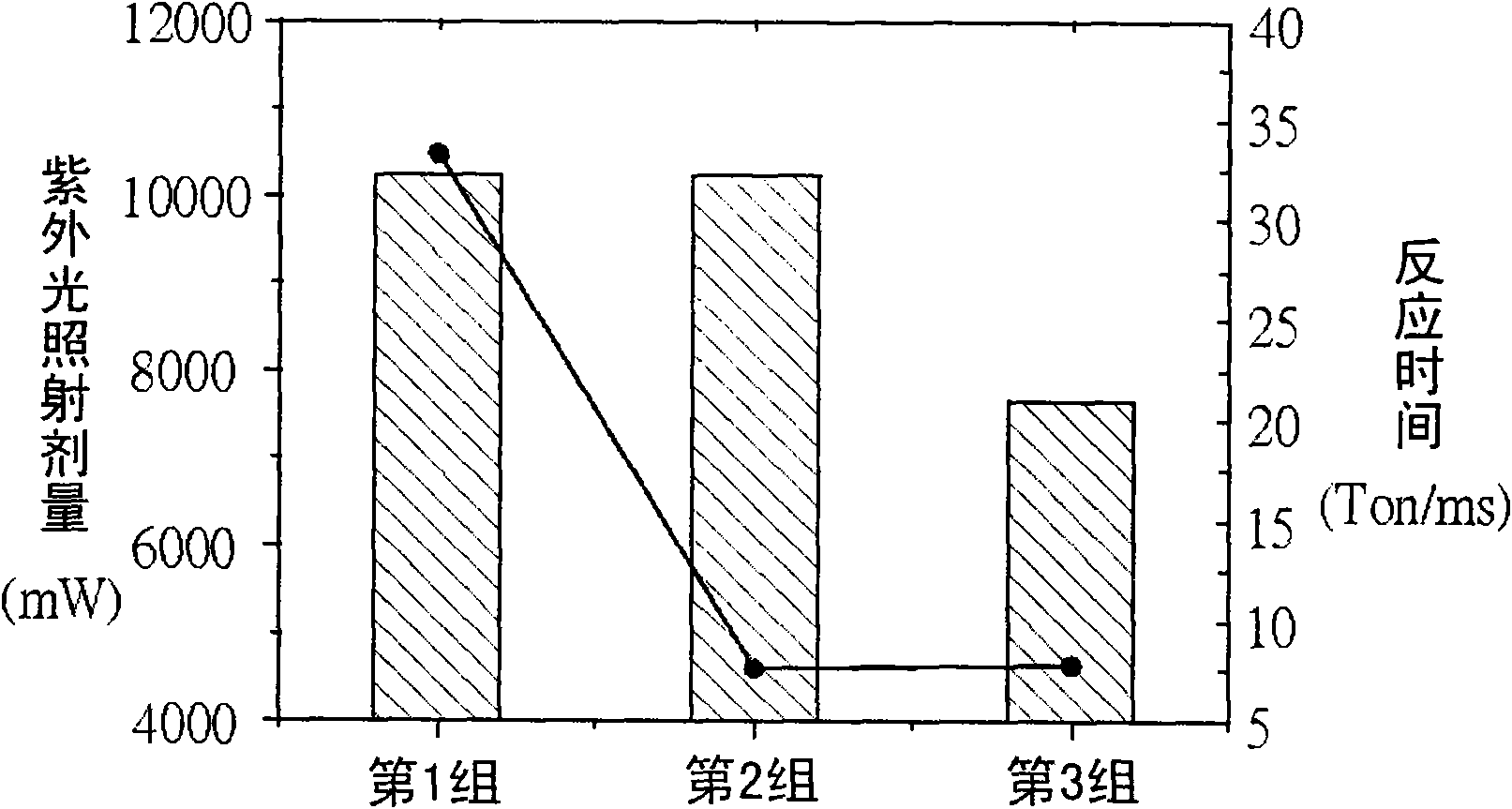
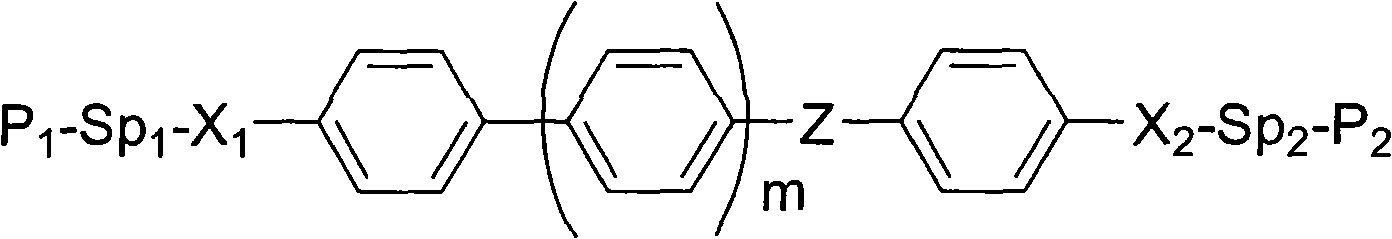
Polymerisable monomer applied to display panel and liquid crystal material
A technology for polymerizing monomers and display panels, which is applied in liquid crystal materials, instruments, organic chemistry, etc., and can solve the problems that the orientation ability of polymer film liquid crystal molecules still needs to be improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Each substituent in the chemical formula of the above-mentioned polymerizable monomer is replaced as follows: m=0, "Z" is a carboxyl group, "X 1 ","X 2 "," Sp 1 " and " Sp 2 "respectively a single key, "P 1 " and "P 2 "Respectively a group IV, that is, compound A. "Y" in the group IV (that is, "R" on the figure) is a hydrogen atom, a methyl group, a fluorine atom, a trifluoromethyl group and a phenyl group.
[0044]
[0045] From the structural point of view, the dihedral angle of the central hard core structure of compound A is very small, and its ultraviolet light absorption wavelength is substantially less than 300nm. On the other hand, the synthetic method of the polymerizable monomer of the present embodiment is represented as follows with chemical reaction formula:
[0046]
[0047] After about 4 mmol of hydroquinone, about 1 mmol of 4-hydroxybenzoic acid (4-hydroxybenzoic acid), about 0.1 mmol of dimethylaminopyridine (DMAP) and 50 ml of dehydrated Put ...
Embodiment 2
[0050] Each substituent in the chemical formula of the above-mentioned polymerizable monomer is replaced as follows: m=1, "Z" is a carboxyl group, "X 1 ","X 2 "," Sp 1 " and " Sp 2 "respectively a single key, "P 1 " and "P 2 "Respectively a group IV, that is, compound B. "Y" in the group IV (that is, "R" on the figure) is a hydrogen atom, a methyl group, a fluorine atom, a trifluoromethyl group and a phenyl group.
[0051]
[0052] From the structural point of view, the dihedral angle of the central hard core structure of compound B is very small, and its ultraviolet light absorption wavelength is substantially less than 300nm. On the other hand, the synthetic method of the polymerizable monomer of the present embodiment is represented as follows with chemical reaction formula:
[0053]
[0054] Add about 4 mmol of 4,4'-dihydroxybiphenyl (4,4'-Dihydroxybiphenyl), about 1 mmol of 4-hydroxybenzoic acid and about 0.1 mmol of DMAP and 50 ml of dehydrated tetrahydrofuran...
Embodiment 3
[0057] Each substituent in the chemical formula of the above-mentioned polymerizable monomer is replaced as follows: m=0, "Z", "X 1 ","X 2 "," Sp 1 " and " Sp 2 "respectively a single key, "P 1 " and "P 2 "Respectively a group IV, that is, compound C. "Y" in the group IV (that is, "R" on the figure) is a hydrogen atom, a methyl group, a fluorine atom, a trifluoromethyl group and a phenyl group.
[0058]
[0059] From the structural point of view, the dihedral angle of the central hard core structure of compound C is very small, and its ultraviolet light absorption wavelength is substantially less than 300nm. The synthetic method of the polymerizable monomer of the present embodiment is expressed as follows with chemical reaction formula:
[0060]
[0061] Put about 4.14 mmoles of 4,4'-dihydroxybiphenyl into a 250mL double-necked bottle, pump air with a vacuum deoxygenation and dewatering device, and fill nitrogen three times, then connect nitrogen to the dropping tu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com