Preparation and application of fluororesins with low ion exchange capacity
An ion exchange, fluororesin technology, applied in the direction of ion exchange, cation exchange, electrical components, etc., can solve the problem of mechanical properties, heat resistance, chemical corrosion resistance, durability reduction, fracture, chemical bond position and fracture can not be accurately controlled And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085]Embodiment 1 (perfluorocarbon solvent system, fluorine-containing organic peroxide is made initiator)
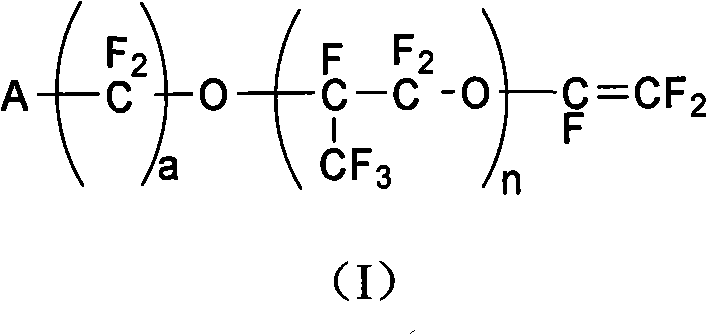
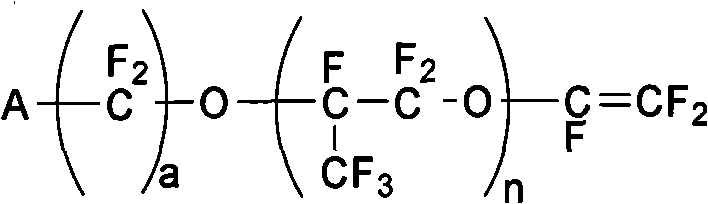
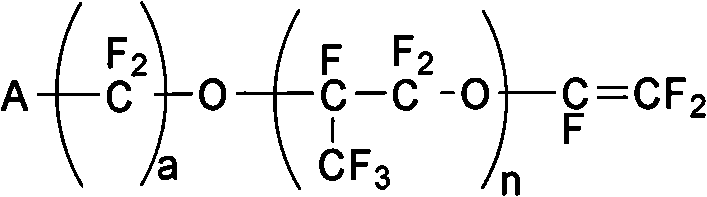
[0086] Polymerization monomers are selected from tetrafluoroethylene and ethylene; vinyl ether monomer: methyl ester group-perfluoropropoxy ethyl isopropyl vinyl ether (molecular formula is CF 2 =CFO-CF 2 (CF 3 ) CFOCF 2 CF 2 COOCH 3 (i.e. n=1, a=2, A=-COOCH 3 ), molecular weight is 422g / mol, preparation reference USP.4578512 preparation)
[0087] Clean and vacuumize the 10L stainless steel autoclave, fill it with high-purity nitrogen and replace it three times until the oxygen content is controlled below 10ppm, vacuumize to -0.1MPa, and add 6L of perfluorocarbon solvent (the perfluorocarbon Solvent is HFC225), 400g carbomethoxy-perfluoropropoxy ethyl isopropyl vinyl ether (molecular formula is CF 2 =CFO-CF 2 (CF 3 ) CFOCF 2 CF 2 COOCH 3 ), stirred and heated up to 40°C, after the temperature of the system was constant, a metering pump was used to add 40ml ...
Embodiment 2
[0093] Embodiment 2: (solution polymerization, fluorocarbon solvent, perfluoroalkyl acyl peroxide initiator)
[0094] Polymerization monomers are selected from tetrafluoroethylene and ethylene; two kinds of vinyl ether monomers (one molecular formula is CF 2 =CFO-CF 2 CF(CF 3 )O-CF 2 CF 2 COOCH 3 (i.e. n=1, a=2, A=-COOCH 3 ), molecular weight 422g / mol, its 2 molecular formula is CF 2 =CFO-CF 2 CF(CF 3 )O-CF 2 CF 2 CF 2 COOCH 3 (i.e. n=1, a=3, A=-COOCH 3 ), molecular weight 472g / mol, carry out solution radical copolymerization.
[0095] The 10L stainless steel autoclave was cleaned and fully dried, then evacuated, filled with nitrogen and replaced three times until the oxygen content was controlled below 10ppm, then evacuated to -0.1MPa, and 6.0L perfluorocarbon solvent (the perfluorocarbon solvent was Perfluorodimethylcyclobutane), 230g of perfluoroalkenyl ether monomer 1 and 250g of perfluoroalkenyl ether monomer 2 were added to the reaction kettle, stirred and ...
Embodiment 3
[0099] Embodiment 3 (suspension polymerization, persulfate is initiator, and water is as dispersion medium)
[0100] Polymerization monomers choose tetrafluoroethylene and ethylene; vinyl ether monomers: CF 2 =CFO-CF 2 CF(CF 3 )O-CF 2 CF 2 COOCH 3 , molecular weight 422g / mol.
[0101] Clean and vacuumize the 10L stainless steel autoclave, fill it with high-purity nitrogen and replace it three times until the oxygen content is below 10ppm, then vacuumize to -0.1MPa, and add 3.2g of perfluoropropoxy ammonium carboxylate ( Molecular formula CF 3 CF 3 CF 2 OCFCF 3 CF 2 -OCFCF 3 COONH 4 ) of pure water 6L, 150g vinyl ether monomer (CF 2 =CFO-CF 2 CF(CF 3 )O-CF 2 CF 2 COOCH 3, molecular weight 422g / mol), heat up to 70°C, feed tetrafluoroethylene, ethylene mixed gas (mixing ratio: tetrafluoroethylene: ethylene = 50: 50) to 4.0MPa, use metering pump to continuously add concentration It is 0.0007mol / L potassium persulfate solution, and the reaction begins. The react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melt flow index | aaaaa | aaaaa |
| Monofilament strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com