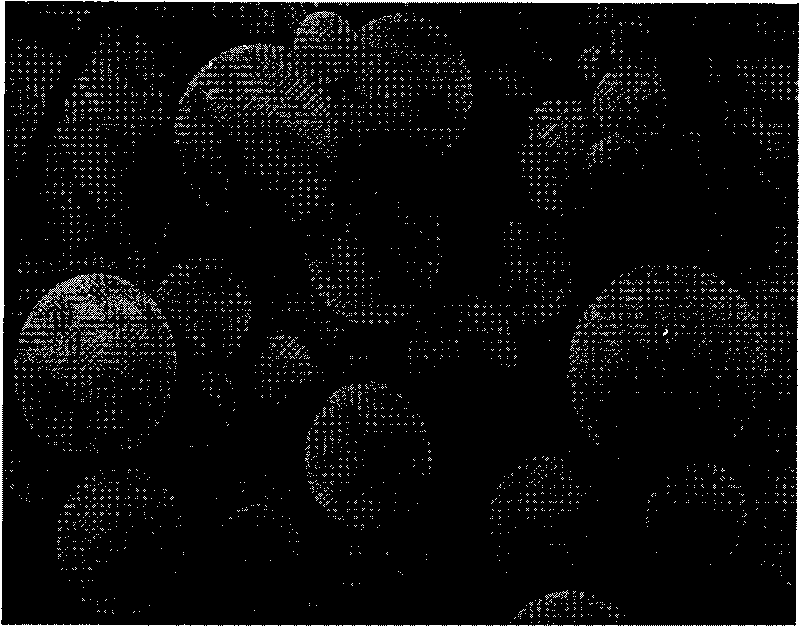Naltrexone long-acting sustained-release preparation with no need of coating and preparation method thereof
A technology of sustained-release preparations and naltrexone, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve toxic and side effects, residues, and short-term Months, no more than half a year, etc., to achieve a smooth surface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
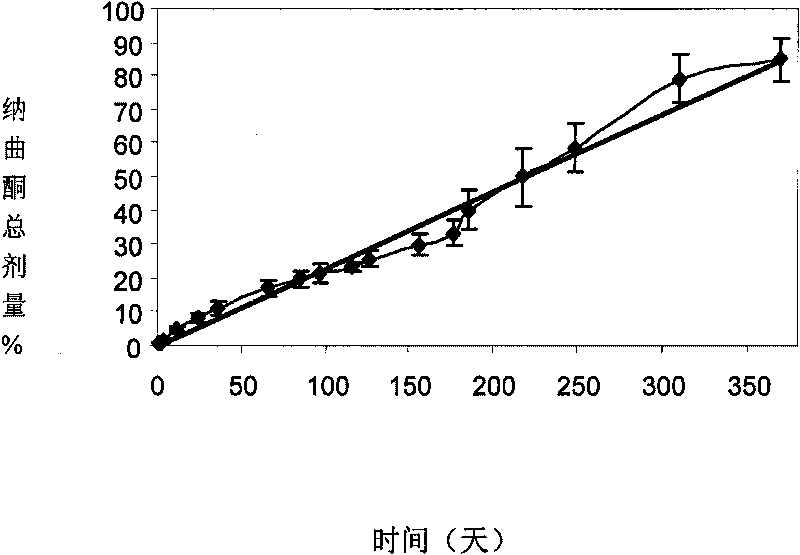
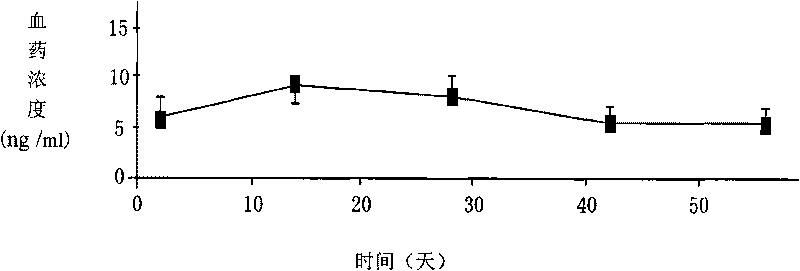
[0043] A long-acting sustained-release agent of naltrexone for long-acting anti-relapse treatment after detoxification of opioid addiction patients.
[0044] Using the W / O / W solvent volatilization method, 10g of racemic polylactic acid (the average molecular weight of polylactic acid is between 50,000 and 400,000 daltons and the intrinsic viscosity is 0.53dL / g) is dissolved in 100ml of two Then add 0.55g of stannous zincate as a catalyst and ultrasonically shake to form colostrum; then add the colostrum solution and 20g of naltrexone base to 1000ml, 1% polyvinyl alcohol (hydrolysis degree 87- 89%, 4% aqueous solution viscosity coefficient 40-46cP at 20℃) in the solution, high-speed stirring (1,500-2000rpm) until the methylene chloride is completely volatilized, then filter out the naltrexone-polylactic acid polymer, and wash with distilled water 3 Next, dehumidify and dry at 30°C to obtain naltrexone-polylactic acid microspheres. The diameter of the microspheres is between 20-200...
Embodiment 2
[0046] A long-acting sustained-release agent of naltrexone for the treatment of alcohol addiction patients.
[0047] Using the W / O / W solvent volatilization method, 10g of racemic polylactic acid (the average molecular weight of polylactic acid is between 50,000 and 300,000 Daltons, and the intrinsic viscosity is 0.53dL / g), dissolved in 100ml of two Then add 0.5g of stannous zincate as a catalyst and ultrasonically shake to form colostrum; then add 1600ml of colostrum solution and 10g of naltrexone base, 1% polyvinyl alcohol (hydrolysis degree 87- 89%, 4% aqueous solution viscosity coefficient 40-46cP at 20℃) in the solution, high-speed stirring (1,500-2000rpm) until the methylene chloride is completely volatilized, and then filtered to separate the naltrexone-polylactic acid polymer, with distilled water Wash 3 times, dehumidify and dry at 30°C to obtain naltrexone-polylactic acid microspheres. The diameter of the microspheres is between 20-200μm and shows a normal distribution (...
Embodiment 3
[0049] A long-acting sustained-release agent of naltrexone for the treatment and prevention of Alzheimer's disease.
[0050] Using the W / O / W solvent volatilization method, 10g of racemic polylactic acid (the average molecular weight of polylactic acid is between 5-25 million Daltons and the intrinsic viscosity is 0.53dL / g) is dissolved in 100ml of two Then add 0.4g of stannous zincate as a catalyst and ultrasonically shake to form colostrum; then add 1000ml of colostrum solution and 12g of naltrexone base to 1% polyvinyl alcohol (hydrolysis degree 87- 89%, 4% aqueous solution viscosity coefficient 40-46cP at 20℃) in the solution, high-speed stirring (1,500-2000rpm) until the methylene chloride is completely volatilized, and then filtered to separate the naltrexone-polylactic acid polymer, with distilled water Wash 3 times, dehumidify and dry at 30°C to obtain naltrexone-polylactic acid microspheres. The diameter of the microspheres is between 20-200μm and shows a normal distribut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com