Polyether-loaded N-heterocyclic carbene-palladium compound and preparation method and application thereof
A technology of heterocyclic carbene and palladium compounds, applied to polyether-supported N-heterocyclic carbene palladium compounds and their preparation, and the application field in the production of aryl carboxylates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
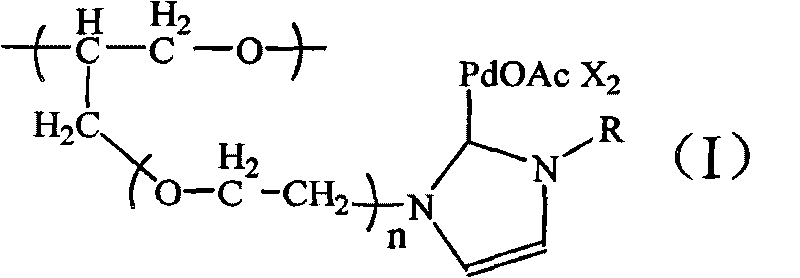
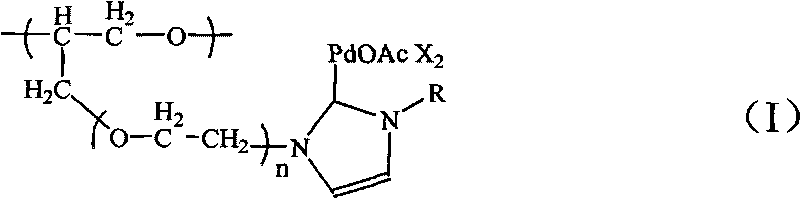
[0029] N-heterocyclic carbene palladium compound supported by polyether, the molecular formula is:
[0030]
[0031] in:
[0032] R=CH 3 ; m=10; n=1; X=Br - (1); R=CH(CH 3 ) 2 ; m=20; n=5; X=Br - (10);
[0033] R=CH 3; m=15; n=1; X=Br - (2); R=C 6 h 11 (cyclohexyl); m=10; n=1; X=Br - (11);
[0034] R=CH 3 ; m=20; n=1; X=Cl - (3); R=C 6 h 11 (cyclohexyl); m=30; n=3; X=Br - (12);
[0035] R=CH 3 ; m=25; n=1; X=Br - (4); R=C 5 h 4 N(pyridine); m=15; n=2; X=1 - (13);
[0036] R=CH 3 ; m=30; n=1; X=Br - (5); R=C 5 h 4 N(pyridine); m=25; n=3; X=Br - (14);
[0037] R=o-C 6 h 3 (CH 3 ) 2 ; m=15; n=1; X=Br - (6); R=C 6 h 7 N 2 (Dimethylpyrimidinyl); m=15; n=1; X=Br - (15);
[0038] R=o-C 6 h 3 (CH 3 ) 2 ; m=15; n=3; X=PF 6 - (7); R=C 6 h 7 N 2 (Dimethylpyrimidinyl); m=20; n=6; X=PF 6 - (16);
[0039] R=o-C 6 h 3 (CH 3 ) 2 ; m=15; n=5; X=Br - (8); R=C 2 h 5 ; m=10; n=10; X=Br - (17);
[0040] R=CH(CH 3 ) 2 ; m=15; n=2; X=Br...
Embodiment 2
[0042] Preparation of polyether-supported N-methyl-N'-oxyethylimidazolcarbene palladium compound (1):
[0043] 1) Add 1mol polyepichlorohydrin (average degree of polymerization m=15), 15mol N-methyl-N'-hydroxyethylimidazolium bromide, 15mol sodium hydride and 50ml N,N-dimethyl The formamide was stirred and dissolved, and stirred and reacted at 80°C for 48 hours; after the reaction was completed, water was added to precipitate a light yellow solid, which was washed with acetone and ether in turn, and dried in vacuum to obtain a hard solid, which was polyether-supported N-methyl-N '-Oxyethyl imidazolium bromide.
[0044] 2) Take the prepared polyether supported N-methyl-N'-oxyethyl imidazolium bromide, 15mol Pd(OAc) 2 15mol of sodium hydride was added to 50ml of N,N-dimethylformamide, stirred and refluxed at 100°C for 12h in a nitrogen atmosphere; after the reaction was completed, it was filtered, and the filter cake was washed with acetone and ether in turn, and vacuum-dried t...
Embodiment 3
[0046] Preparation of polyether-supported N-o-dimethylphenyl-N'-oxyethylimidazolcarbene palladium compound (6):
[0047] 1) Add 1mol polyepichlorohydrin (average degree of polymerization m=10), 10mol N-o-dimethylphenyl-N'-hydroxyethyl imidazolium bromide, 10mol sodium tert-butoxide and 50ml N , N-dimethylformamide was stirred and dissolved, and stirred and reacted at 100°C for 32 hours; after the reaction was completed, water was added to precipitate a light yellow solid, which was washed with acetone and ether in turn, and a hard solid was obtained after vacuum drying, which was polyether-loaded N-o-dimethylphenyl-N'-oxyethylimidazolium bromide.
[0048] 2) Load N-o-dimethylphenyl-N'-oxyethyl imidazolium bromide, 10mol PdCl 2 Add 10mol sodium tert-butoxide to 50ml N,N-dimethylformamide, stir and reflux at 110°C for 10h in a nitrogen atmosphere; filter after the reaction, wash the filter cake with acetone and ether in turn, and vacuum dry to obtain Product (6), yield 73.2%. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com