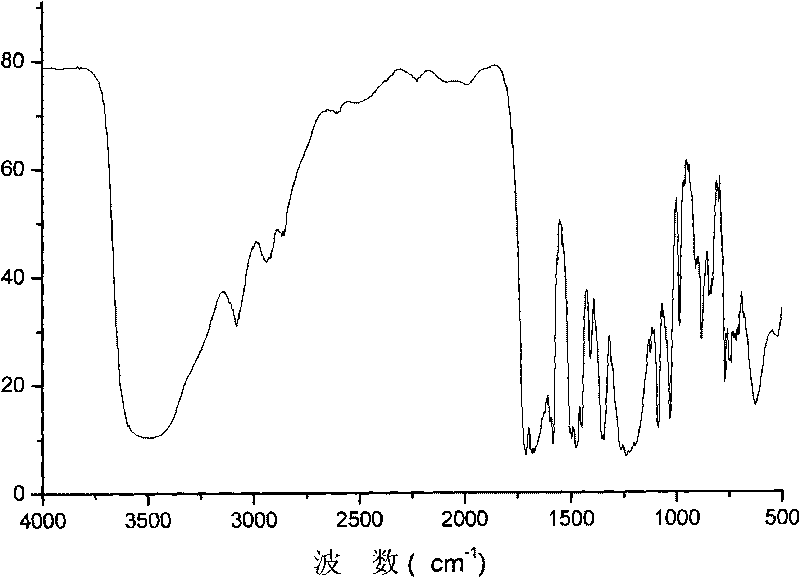
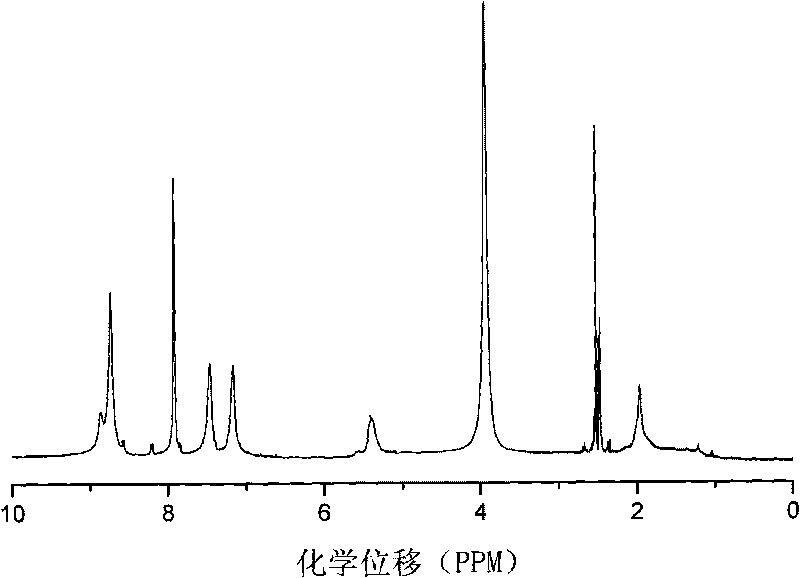
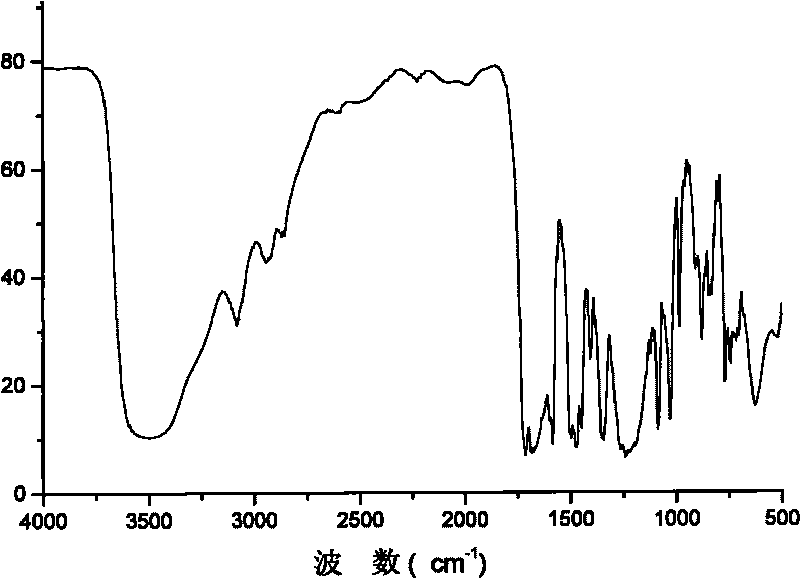
Segmented copolymer of fully sulfonated polymide and partly sulfonated polybutadiene and preparation method thereof
A technology of fully sulfonated polyimide and sulfonated polyimide, applied in the field of functional polymer materials, can solve problems such as high price and restrictions, achieve sufficient mechanical strength, improve processability, and high proton conductivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (a) Dissolve 3.25g of carboxy-terminated polybutadiene in 30g of toluene, add 3g of thionyl chloride and stir evenly; rise to 60°C for 3 hours under nitrogen protection, then rise to 110°C for 12 hours After the reaction is finished, cool down, and remove the remaining thionyl chloride by distillation under reduced pressure, so as to obtain the toluene solution of polybutadiene with acyl chloride terminal;
[0031] (b) Add 4g 4,4'-diaminodiphenyl ether to a round-bottomed flask placed in an ice bath, and slowly add 20g of concentrated sulfuric acid with a mass concentration of 98%; after the solid is completely dissolved, add 20g of The mass concentration is 50% oleum; the mixed solution was stirred in an ice bath for 2 hours, and then raised to 80° C. to continue the reaction for 10 hours; the reaction solution was cooled and slowly poured into 40 g of ice-water mixture to obtain a large amount of white Precipitation; dissolve the precipitate obtained by filtration in ...
Embodiment 2
[0039] (a) Dissolve 6.5g of carboxy-terminated polybutadiene in 40g of toluene, add 6g of thionyl chloride and stir evenly; rise to 70°C for 2 hours under nitrogen protection, then rise to 100°C for 15 hours After the reaction is finished, cool down, and remove the remaining thionyl chloride by distillation under reduced pressure, so as to obtain the toluene solution of polybutadiene with acyl chloride terminal;
[0040] (b) Add 6g of 4,4'-diaminodiphenyl ether to a round-bottomed flask placed in an ice bath, and slowly add 30g of concentrated sulfuric acid with a mass concentration of 98%; after the solid is completely dissolved, add 30g of it dropwise The mass concentration is 50% oleum; the mixed solution was stirred in an ice bath for 2 hours, and then raised to 90° C. to continue the reaction for 8 hours; the reaction solution was cooled and slowly poured into 60 g of ice-water mixture to obtain a large amount of white Precipitation; dissolve the precipitate obtained by f...
Embodiment 3
[0048] (a) Dissolve 9.75g of carboxyl-terminated polybutadiene in 50g of toluene, add 9g of thionyl chloride and stir evenly; rise to 80°C for 1 hour under nitrogen protection, then rise to 90°C for 18 hours After the reaction is finished, cool down, and remove the remaining thionyl chloride by distillation under reduced pressure, so as to obtain the toluene solution of polybutadiene with acyl chloride terminal;
[0049] (b) Add 8g of 4,4'-diaminodiphenyl ether to a round-bottomed flask placed in an ice bath, and slowly add 40g of concentrated sulfuric acid with a mass concentration of 98%; after the solid is completely dissolved, add 40g of it dropwise The mass concentration is 50% oleum; the mixed solution was stirred in an ice bath for 2 hours, and then raised to 100° C. to continue the reaction for 6 hours; the reaction solution was cooled and slowly poured into 80 g of ice-water mixture to obtain a large amount of white Precipitation; dissolve the precipitate obtained by ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com