Use of human interleukin 32 in preparation of medicament for treating or preventing influenza A virus infection
A technology of influenza virus infection and human interleukin, applied in antiviral agents, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of undetectable IL-32 and undetectable IL-32 synthesis, and achieve The effect of avoiding toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Plasmid construction and identification
[0031] The cDNA was obtained by reverse transcription PCR after extracting the mRNA of Jurkat cells, and used it as a template to amplify the target fragment with the cloning primers of each indirect isomer. The vector Pcmv-Tag2A (Sigma Company) was digested with MluI and HindIII, purified and recovered, and ligated in a water bath at 16°C overnight. Transformed into Escherichia coli DH5α by electroporation (China Type Culture Collection-Wuhan University Collection Center, common Escherichia coli), spread it on the ampicillin-resistant LB culture plate, culture at 37°C for 12-16h, and select single clones Perform PCR and enzyme digestion identification, select 2 clones for sequencing, and identify whether the sequence and coding frame of the target fragment are correct.
[0032] A. DNA fragment recovery, glass milk (glass-milk) method
[0033] 1. Take the PCR product, add 1 / 10 volume of 10X loading buffer, and electrophoresis ...
Embodiment 2
[0088] Mammalian cell culture and transfection
[0089] A. Mammalian cell culture
[0091] 1) Take the cells out of the liquid nitrogen tank and quickly thaw them in a 37°C water bath, shaking the cryopreservation tube constantly during this period to make the cell liquid evenly heated;
[0092] 2) Transfer the thawed cells into a 25ml cell bottle, and then add 3ml of corresponding DMEM medium (manufacturer GIBCO, the same below);
[0093] 3) Placed at 37°C, 5% (volume ratio) of CO 2 Cultivated in an incubator;
[0094] 4) After the cells adhere to the wall, replace with new DMEM medium.
[0095] 2. Cell passage
[0096] 1) The flat space where the cells grow is full, and the DMEM medium is discarded;
[0097] 2) Add 8ml of D-Hank's buffer, wash the cells, and remove the washing solution;
[0098] 3) Add 1ml of trypsin and place at 37°C for 5 minutes;
[0099] 4) Add 4ml DMEM medium to neutralize trypsin;
[0100] 5) Aspirate 2 / 3 of the cell ...
Embodiment 3
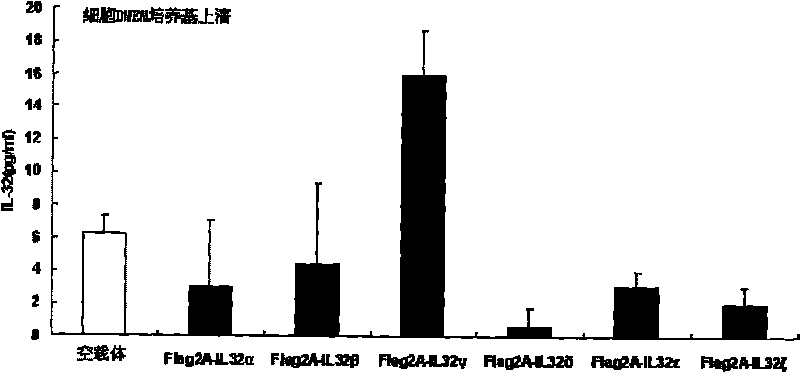
[0120] A. EILSA method to detect IL-32 protein expression
[0121] 1. Serum, cell culture supernatant or cell lysate samples were diluted 1:50 with PBS (pH 7.4), 100 μl was added to the wells of the ELISA plate, and coated at 37°C for one hour or overnight at 4°C.
[0122] 2. Wash the ELISA plate 5 times with PBS (try to pat the liquid clean after each wash to ensure that it is as clean as possible).
[0123] 3. Prepare 3% (volume ratio) PBST skimmed milk powder, dissolve in PBST, add 100 μl per well to the ELISA plate, and block for one hour at 37°C or overnight at 4°C.
[0124] 4. Wash more than 5 times with PBST.
[0125] 5. Peptide immunization The IL-32 antibody prepared from rabbits was diluted 1:500 with PBST, added 100 μl to each well, and kept at 37° C. for one hour.
[0126] 6. Wash more than 5 times with PBST.
[0127] 7. Horseradish peroxidase-coupled goat anti-rabbit IgG was diluted 1:5000 with PBST, and kept at 37°C for half an hour.
[0128] 8. Wash more tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com