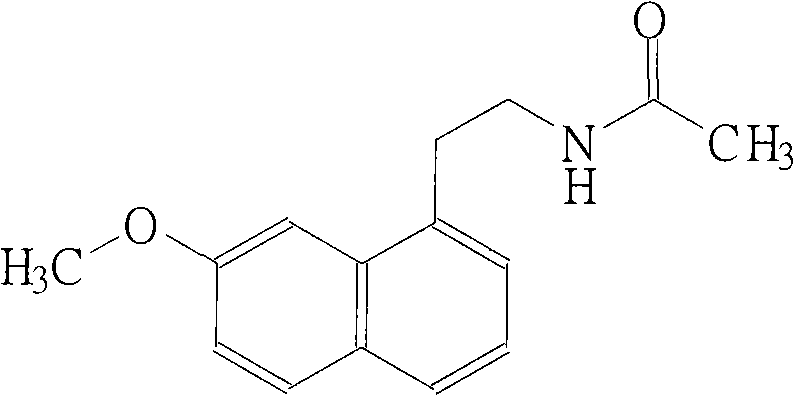
Preparation method of agomelatine I type crystal
A crystal, type I technology, applied in the field of preparation of agomelatine type I crystal, can solve problems such as weight change, withdrawal syndrome, sexual dysfunction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0027] Preparation of 7-methoxy-1-naphthylacetic acid
[0028] Dissolve 40 g of sodium hydroxide in 1000 ml of water, add 1000 ml of 95% ethanol, and mix well. Then, 50 g of (7-methoxy-1-naphthyl)-ethyl acetate was added to the above mixed solution and stirred at room temperature for 3 hours. The reaction was stopped and the ethanol was evaporated under reduced pressure to obtain a brownish red liquid. After washing with ethyl acetate 300ml X 2, 30ml of 95% ethanol was added to the water layer. Under stirring, concentrated hydrochloric acid was added dropwise to adjust the pH to 2, and a large amount of light brown was precipitated. solid. The product was filtered and dried to obtain 32 g, mp154-156°C, the content determined by HPLC was 98.48%, and the yield was 72%.
[0029] Reference Example 4: Preparation of 7-methoxy-1-naphthylacetamide
[0030] Add 50 g of 7-methoxy-1-naphthyl acetic acid to 750 ml of dichloromethane, heat to dissolve, and slowly add thionyl chloride dropwise...
Embodiment 7
[0038] Preparation of N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide (agomelatine)
[0039] Dissolve 40g of 2-(7-methoxy-1-naphthyl)ethylamine in 250ml of pyridine, and heat to 40°C to dissolve it. Under ice-bath cooling and stirring, 21.9 g of acetyl chloride was slowly added dropwise. After dripping, remove the ice bath and stir at room temperature for 30 minutes. Then the reaction solution was poured into 300ml ice water, while vigorously stirring, a large amount of white precipitate was precipitated, stirring was continued for 1 hour, filtered, and the filter cake was washed with 200ml×2 water to obtain 48g of crude product (the obtained crude product was used in the following preparation examples).
[0040] 1 H NMR(400MHZ, CDCl 3 ): δ7.77-7.15(m 6H,); δ5.61(s, 1H,); δ3.99(s, 3H,); δ3.62(m, 2H,); δ3.25(t, 2H ,); δ 1.95 (s, 3H,) is consistent with literature reports (J. Med. Chem, 1994, 37(20), 3231-3239.
[0041] Preparation examples
Embodiment 1
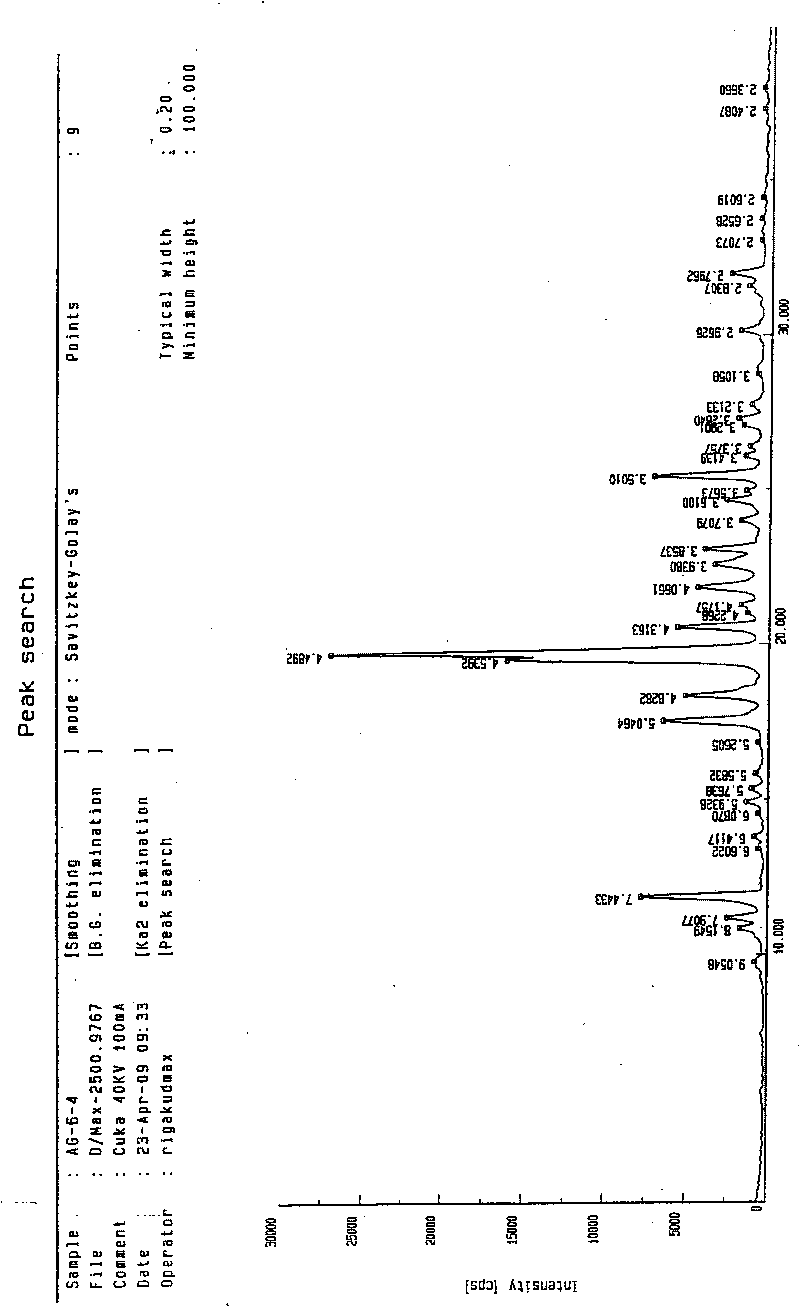
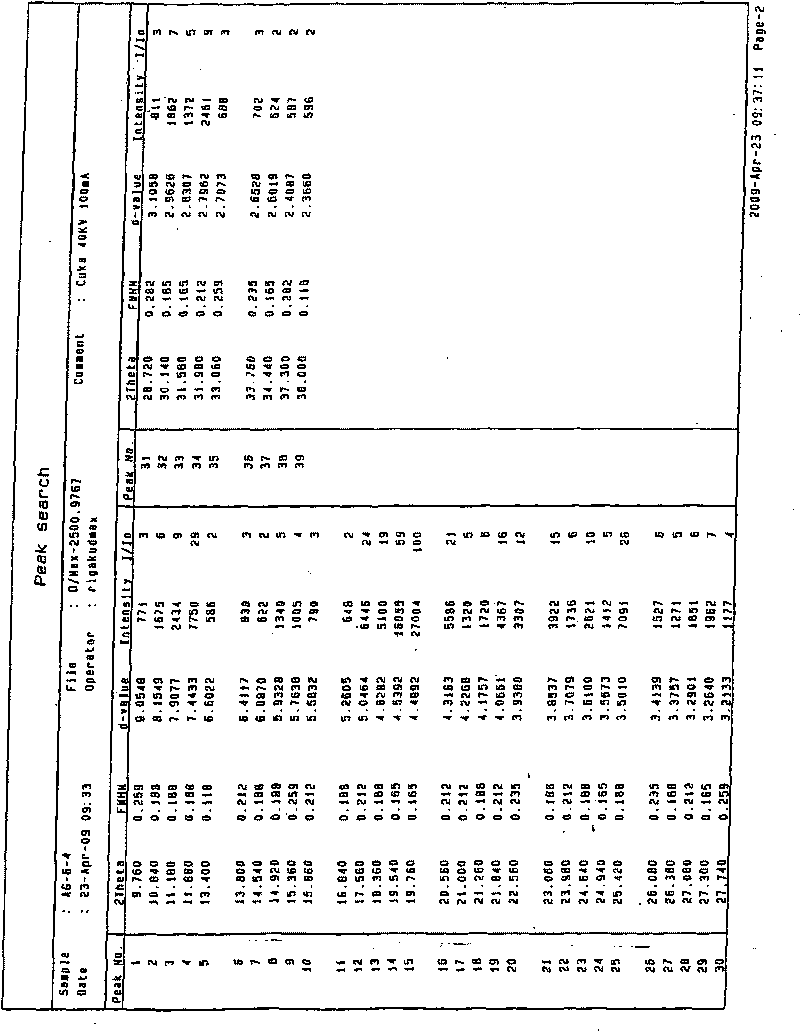
[0043] Add 2g of agomelatine phase product to 10ml of absolute ethanol to dissolve, filter, and slowly drip the filtrate into 200ml of distilled water with stirring at room temperature. After the addition is complete, stir at room temperature for 30 minutes, filter, wash the filter cake with distilled water, and vacuum dry for 10 hours. The yield is 88.5% and the content is 99.4%. The single crystal data is consistent with the type I data described in the literature (ActaCryst, 1994, C50, 907-910.); see the X-ray powder diffraction pattern figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com