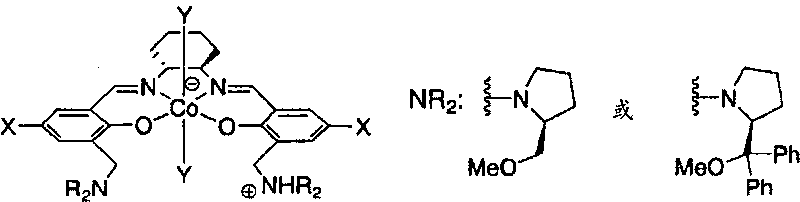
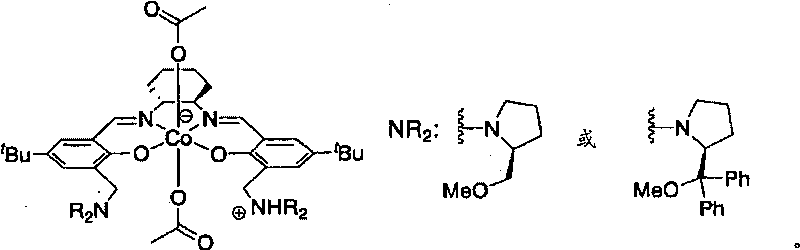
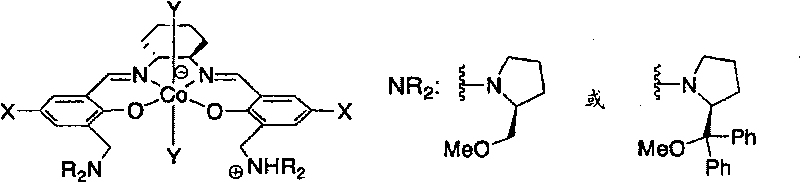Epoxide-carbon dioxide stereoselective alternating copolymer
A technology of epoxy and carbon dioxide, which is applied in the field of preparing polycarbonate with high stereoregularity, can solve the problem of high price and achieve the effect of high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The epoxide compound that can be used as a monomer raw material in the production method of the present invention includes any of chiral epoxides and meso epoxides. Specifically, an epoxide represented by the following formula may be included.
[0036]
[0037] (In the above formula, R 1 and R 2 Under the condition that they are not hydrogen atoms at the same time, they can be the same or different, and R 1 and R 2 independently hydrogen atom, halogen atom, substituted amino (-NR 3 R 4 ), cyano, linear or branched C 1 ~C 20 Alkyl, C 2 ~C 20 Alkenyl or C 2 ~C 20 Alkynyl, C 4 ~C 10 Cycloalkyl, C 6 ~C 40 Aryl or C 7 ~C 40 Arylalkyl, or, R 1 and R 2 can also together form saturated or unsaturated C 4 ~C 10 Alicyclic group; the aryl part in the above-mentioned aryl and arylalkyl and the above-mentioned alicyclic group can also be selected from halogen atoms, straight chain or branched C 1 ~C 20 Alkyl, C 2 ~C 20 Alkenyl or C 2 ~C 20 Alkynyl, C 4 ...
Embodiment
[0052] The present invention will be described more specifically by the following examples, but the present invention is not limited to these examples.
[0053] The compounds obtained in this example 1 The measurement of the H NMR spectrum was performed with JNM-ECP500 (500 MHz) manufactured by JEOL Corporation.
[0054] Molecular weight determination of optically active polycarbonate, using a high-performance liquid chromatography system (DG660B·PU713·UV702·RI704·C0631A) manufactured by GL Sciences and 2 KF-804F columns manufactured by SHODEX, using tetrahydrofuran as the eluent (40° C., 1.0 mL / min), measured in terms of polystyrene standard as a standard, processed and obtained by analysis software (EZChrom Elite, manufactured by Scientific Software Co., Ltd.).
[0055] In addition, in this example, the optical purity of the unreacted optically active epoxide was determined based on the enantiomeric excess (%ee) calculated by converting the epoxide into the corresponding cy...
Synthetic example A
[0065] Synthesis Example A: Synthesis of Novel Schiff Base Compounds
[0066] A-1. Synthesis of salcy ligand 1b
[0067]
[0068] Under argon atmosphere, in a Schlenk tube with a capacity of 20 mL, add salicylaldehyde derivative 3 (248 mg, 0.91 mmol) and tetrahydrofuran (10 mL), slowly add (S)-2- (Diphenylmethoxymethyl)pyrrolidine [(S)-4, 450 mg, 1.7 mmol]. After stirring at 25°C for 2 hours, the resulting precipitate was filtered, and the filtrate was concentrated to obtain a salicylaldehyde derivative (S)-5 (384 mg, yield 84%). 1 HNMR (CDCl 3 , 500MHz)d10.29(s, 1H), 7.56-7.50(m, 5H), 7.40-7.29(m, 8H), 4.36(d, J=13.5Hz, 1H), 3.99(dd, J=9.9, 4.1Hz, 1H), 3.68(d, J=13.7Hz, 1H), 2.96(s, 3H), 2.38-2.34(m, 1H), 2.20-2.15(m, 1H), 2.10-2.02(m, 1H ), 1.86-1.80 (m, 1H), 1.46-1.40 (m, 1H), 1.30 (s, 9H), 0.72-0.62 (m, 1H).
[0069]
[0070] The obtained salicylaldehyde derivative (S)-5 (178 mg, 0.39 mmol) was dissolved in ethanol (1.0 mL), dichloromethane (3.0 mL), and (1R,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com