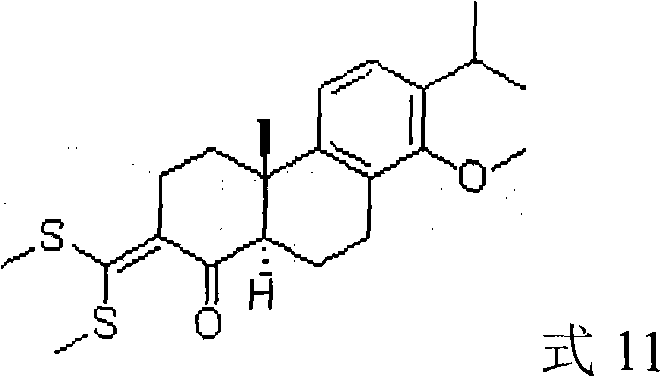
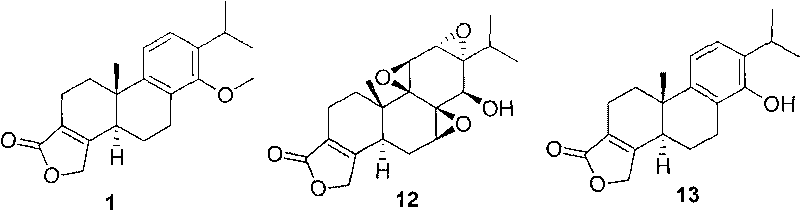
3-substituted-4-ketone-14-methoxyl abietane compounds as well as preparation methods and applications thereof
A technology for methoxyabietane and compounds, which is applied in the field of preparation of refenolactone methyl ether, and can solve problems such as difficulty in taking into account industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
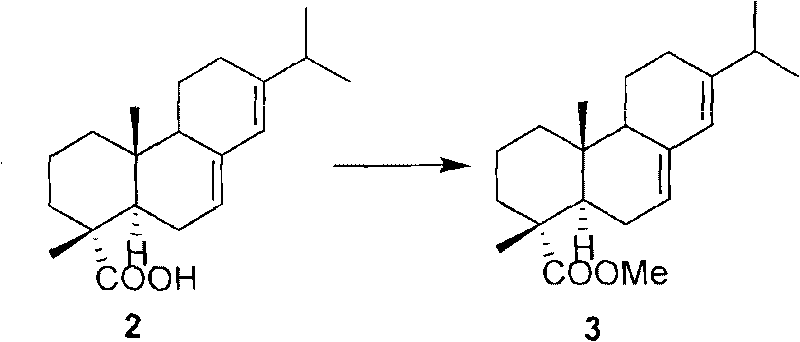
[0053] Embodiment 1: the preparation of the compound (7(8), 13(14)-abietin-18-carboxylate) shown in formula 3
[0054]
[0055] The compound shown in formula 2 (100g, 0.331mol) was dissolved in 500ml of acetone, potassium carbonate (45.7g, 0.331mol) and dimethyl sulfate (31.36ml, 0.331mol) were added at room temperature and reacted for 5 hours, then 50ml of ammonia was added Quench the reaction, spin dry most of the solvent under reduced pressure, add ethyl acetate and saturated saline for extraction, combine the organic layers, wash with water and saturated saline solution, and anhydrous Na 2 SO 4 Dry to obtain compound (colorless oily substance) shown in formula 3,
[0056] 103.55g), the yield was 99%.
[0057] [α] 23 D -65.3 (c 0.49, CHCl 3 )
[0058] 1 H NMR (CDCl 3 , 300MHz) δ=5.77(s, 1H), 5.41(m, 1H), 3.63(s, 3H), 2.22(m, 1H), 1.25(s, 3H), 1.01(d, J=6.9Hz, 3H ), 1.00(d, J=6.9Hz, 3H), 0.82(s, 3H);
[0059] 13 C NMR (CDCl 3 , 100MHz) δ=179.0, 145.3, 135.5, 1...
Embodiment 2
[0061] Example 2: Preparation of the compound shown in Formula 4 (13β, 14β-dihydroxyabietane-7(8)-ene-18-carboxylic acid methyl ester)
[0062]
[0063] Compound (100g, 0.316mol) shown in formula 3 was dissolved in acetone (500ml) and water (500ml), added NMO (N-methylmorpholine-N-oxide) (44.5g, 0.380mol), pyridine ( 30.7g, 0.316mol) and potassium osmate dihydrate (0.23g, 0.0006mol), stirred at reflux for 3 days, added 200ml of saturated sodium bisulfite solution to quench the reaction, and spin-dried most of the solvent under reduced pressure, then added ethyl acetate Ester extraction, combined organic layers, washed with 5% hydrochloric acid, water, saturated saline solution, anhydrous Na 2 SO 4 Dry, filter, and evaporate the filtrate to dryness under reduced pressure to obtain the product. After silica gel column chromatography, the compound represented by formula 4 (white solid, 50 g) was obtained. The raw material (30 g) was recovered, and the yield was 65% based on ...
Embodiment 3
[0068] Example 3: Preparation of the compound shown in Formula 5 (13β-hydroxyabietane-7(8)-ene-14-one-18-carboxylic acid methyl ester)
[0069]
[0070] Method 1: Dissolve the compound shown in formula 4 (46g, 0.131mol) in 500ml carbon tetrachloride, add the dichloromethane solution of diphenyl diselenide (45.5g, 0.144mol) and 2.2M tert-butyl hydroperoxide (143ml, 0.314mol), the reaction system was protected by Ar gas, and after reflux and stirring for 2 hours, 50ml of saturated aqueous solution of sodium bisulfite was added to quench the reaction. , washed with saturated saline solution, anhydrous Na 2 SO 4 Dry, filter, and evaporate the filtrate to dryness under reduced pressure to obtain the product. After silica gel column chromatography, the compound represented by formula 5 (colorless oil, 34.4 g) was obtained with a yield of 75%.
[0071] Method 2: Put the compound shown in Formula 4 (29.2g, 0.083mol) in a 1L three-necked flask, protect the reaction system with ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com