Medicament containing natriuretic peptide gene as well as preparation method and application thereof
A natriuretic peptide and drug technology, applied in the field of gene medicine for the treatment of glaucoma, can solve the problem, which can only be injected into the eye through subconjunctival and vitreous injection, natriuretic peptide has not been widely used, natriuretic peptide gene drug therapy Glaucoma has not yet been reported and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Amplification and cloning of embodiment 1 CNP gene
[0027] 1 material
[0028] 1.1 Plasmids and cell lines
[0029] 1) Lentivector expression systems (pPACKF1 TM Packaging Mix): Purchased from SBI Company in the United States, it is prepared by mixing three plasmids pCDF1-MCS2-EF1-copGFP, pFIV-34N and pVSV-G in a certain proportion.
[0030] 2) 293FT cell line: from Invitrogen Company of the United States, which is preserved in our laboratory.
[0031] 1.2 Tool enzymes and main reagents
[0032] 1) Reagent, Lipofectamine TM 2000 and trypsin (Tyrisin) for digestion: purchased from Invitrogen, USA
[0033] 2) TakaRa One Step RNA PCR Kit (AMV) and diethyl pyrocarbonate (diethypyrocarbonate, DEPC): purchased from Japan TakaRa Company
[0034] 3) Plasmid extraction kit PureYield TM Plasmid Midiprep System: purchased from Promega, USA
[0035] 4) Restriction endonuclease and T4DNA ligase: purchased from MBI Fermentas, USA
Embodiment 2
[0094] Example 2 Construction of recombinant plasmids
[0095] 1 pCDF1-MCS2-EF1-copGFP empty vector and PCR product of CNP gene digested and recovered
[0096] The pCDF1-MCS2-EF1-copGFP empty vector and the CNP gene PCR product were double digested with restriction endonucleases BamH I and EcoR I, respectively. The double enzyme digestion reaction system is as follows, and the reaction condition is 37°C water bath for 4h.
[0097]
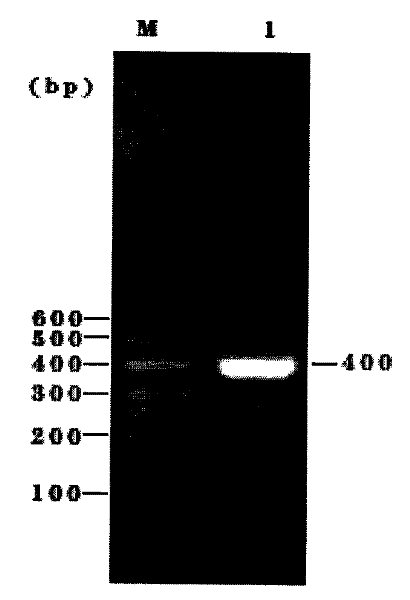
[0098] After the plasmid pCDF1-MCS2-EF1-copGFP was double digested, after 0.7% agarose gel electrophoresis for 30 minutes, the gel at the position of 6749bp was accurately cut, and then the gel was recovered. After double enzyme digestion of the PCR product, after 1.5% agarose gel electrophoresis for 30 minutes, the gel at the position of 410 bp was accurately cut, and then the gel was recovered.
[0099] The digested vector and PCR products were recovered using the gel recovery kit from BioBasic.
[0100] 2 Construction of pCDF1-MCS2-EF1-cop...
Embodiment 3
[0119] Example 3 Packaging of lentivirus
[0120] pPACKF1 mediated by liposome Lipofectamine2000 TM Packaging Plasmid Mix and expression plasmid (empty vector pCDF1-MCS2-EF1-copGFP or recombinant plasmid pCDF1-MCS2-EF1-copGFP-CNP) were co-transfected into 293FT cells to package the virus. Virus packaging flowchart ( Figure 4 ).
[0121] Virus harvest, concentration and titer detection
[0122] 48 and 72 hours after transfection of 293FT cells, the medium supernatant was harvested. Cell debris was removed by centrifugation. Then ultracentrifugation was carried out, and the precipitate was resuspended with PBS, that is, the concentrated lentivirus containing the recombinant plasmid was harvested (the pCDF1-MCS2-EF1-copGFP Vector connected to the CNP gene was packaged into the protein envelope of the virus) FIV-CNP and Lentivirus containing empty vector (packaging of pCDF1-MCS2-EF1-copGFP Vector not connected with CNP gene into viral protein coat) virus stock solution.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com