Method for analyzing arsenic in ore sample
An analysis method and technology of ore samples, which are applied in the direction of material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, can solve problems such as difficulties in arsenic determination, reduce reducing acidity, and improve operations. Conditions, Effects of Good Accuracy and Precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
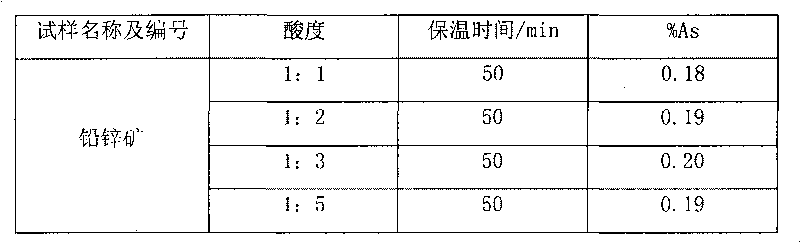
[0017] Weigh 0.5065g of sample into a beaker, add 20ml of nitric acid and 0.5g of potassium chlorate, heat to dissolve for 10min, cool slightly, add 7.5ml of sulfuric acid, continue to heat to dissolve and evaporate to smoke of sulfur trioxide for 5 minutes, after cooling to room temperature, add 30ml of water and 0.1g of copper sulfate, boil to dissolve the salts, and cool slightly. Add 10ml hydrochloric acid, then add sodium hypophosphite to Fe 3+ The yellow color fades away completely, and an excess of 2g is added, boiled, and kept at a slight boil for 50 minutes, so that the arsenic precipitates and coagulates. Knead a small ball with absorbent cotton and stuff it in the funnel, while filling the funnel neck with water. Filter the arsenic precipitate, wash the beaker and the precipitate 4-5 times each with hydrochloric acid (1+3) solution containing sodium hypophosphite, then wash the beaker 3-4 times with 5% ammonium chloride solution, and check the filtered out with pH ...
Embodiment 2
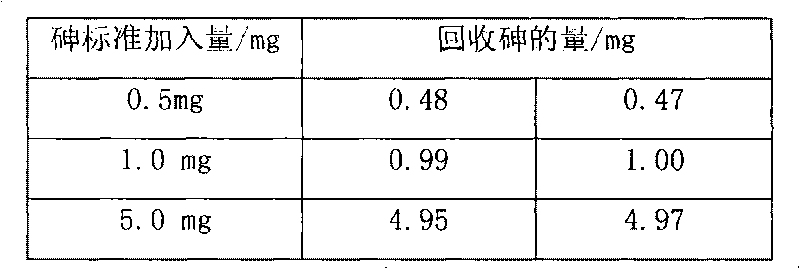
[0019] Weigh 0.1066g of sample into a beaker, add 20ml of nitric acid and 0.5g of potassium chlorate, heat to dissolve for 10min, cool slightly, add 7.5ml of sulfuric acid, continue to heat to dissolve and evaporate to smoke of sulfur trioxide for 5 minutes, after cooling to room temperature, add 30ml of water and 0.1g of copper sulfate, boil to dissolve the salts, and cool slightly. Add 10ml hydrochloric acid, then add sodium hypophosphite to Fe 3+ The yellow color fades away completely, and an excess of 2g is added, boiled, and kept at a slight boil for 50 minutes, so that the arsenic precipitates and coagulates. Knead a small ball with absorbent cotton and stuff it in the funnel, while filling the funnel neck with water. Filter the arsenic precipitate, wash the beaker and the precipitate 4-5 times each with hydrochloric acid (1+3) solution containing sodium hypophosphite, then wash the beaker 3-4 times with 5% ammonium chloride solution, and check the filtered out with pH ...
Embodiment 3
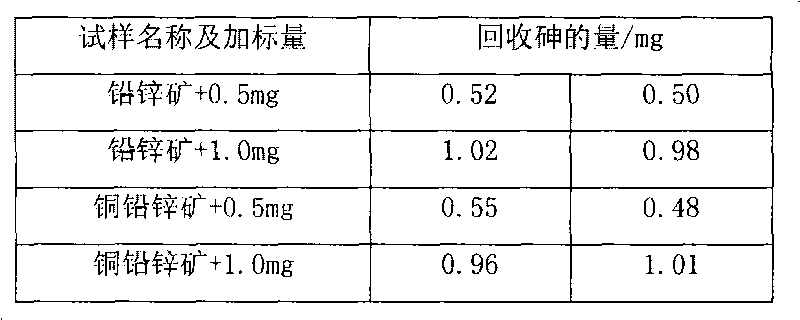
[0021] Weigh 0.1023g of sample into a beaker, add 20ml of nitric acid and 0.5g of potassium chlorate, heat to dissolve for 10min, cool slightly, add 7.5ml of sulfuric acid, continue to heat to dissolve and evaporate to smoke of sulfur trioxide for 5 minutes, after cooling to room temperature, add 30ml of water and 0.1g of copper sulfate, boil to dissolve the salts, and cool slightly. Add 10ml hydrochloric acid, then add sodium hypophosphite to Fe 3+ The yellow color fades away completely, and an excess of 2g is added, boiled, and kept at a slight boil for 50 minutes, so that the arsenic precipitates and coagulates. Knead a small ball with absorbent cotton and stuff it in the funnel, while filling the funnel neck with water. Filter the arsenic precipitate, wash the beaker and the precipitate 4-5 times each with hydrochloric acid (1+3) solution containing sodium hypophosphite, then wash the beaker 3-4 times with 5% ammonium chloride solution, and check the filtered out with pH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com