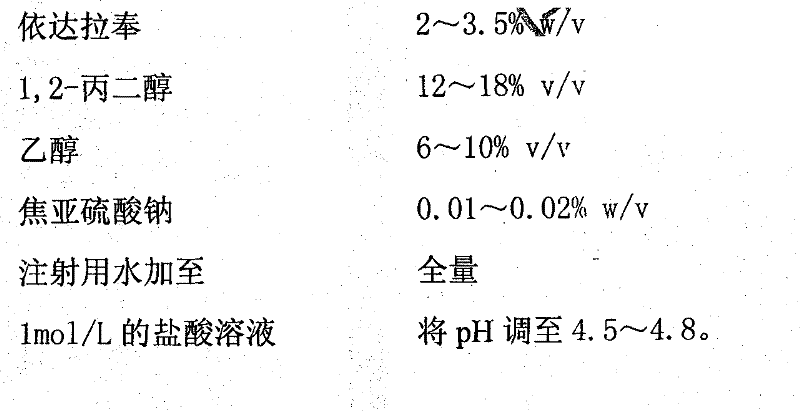
Edaravone-containing injection
A technology for injection and water for injection, which is applied to medical preparations containing active ingredients, medical preparations with non-active ingredients, organic active ingredients, etc. It can solve problems such as errors, contamination of medicinal liquid, and many steps in the preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
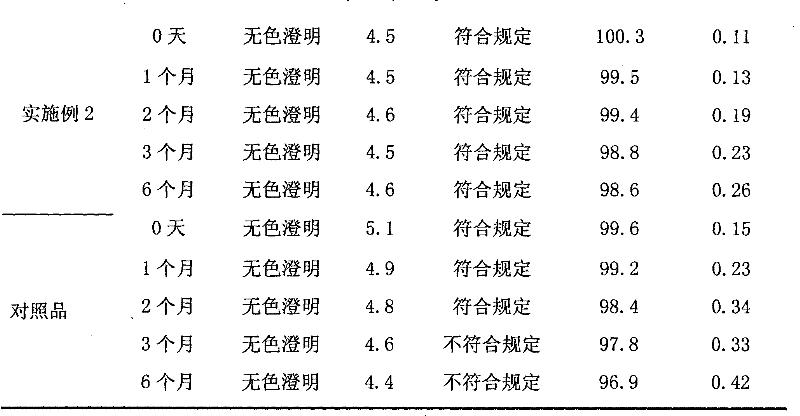
[0047] To specific embodiment 1,2,3,4,5,6 prepared Edaravone injection sample (remove outer packaging) and reference substance (the Edaravone injection that has been listed: trade name " must deposit ", Produced by Nanjing Xiansheng Dongyuan Pharmaceutical Co., Ltd., batch number 20080920) were subjected to high temperature (60°C ± 2°C) influence factor test for 10 days and high temperature light (4500Lx ± 500Lx) influence factor test for 10 days. On the 5th day and the 10th day, samples were taken for testing. The results of the high temperature test are shown in Table 1, and the results of the light test are shown in Table 2.
[0048] Table 1 High temperature test results
[0049] place a study project
[0050] Time Sample Appearance color pH Visible foreign matter Content Related substances
Embodiment 1
[0051] Example 1 Colorless and clear 5.0 Complies with regulations 100.1 0.08
Embodiment 2
[0052] Example 2 Colorless and clear 4.5 Complies with regulations 100.2 0.05
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com