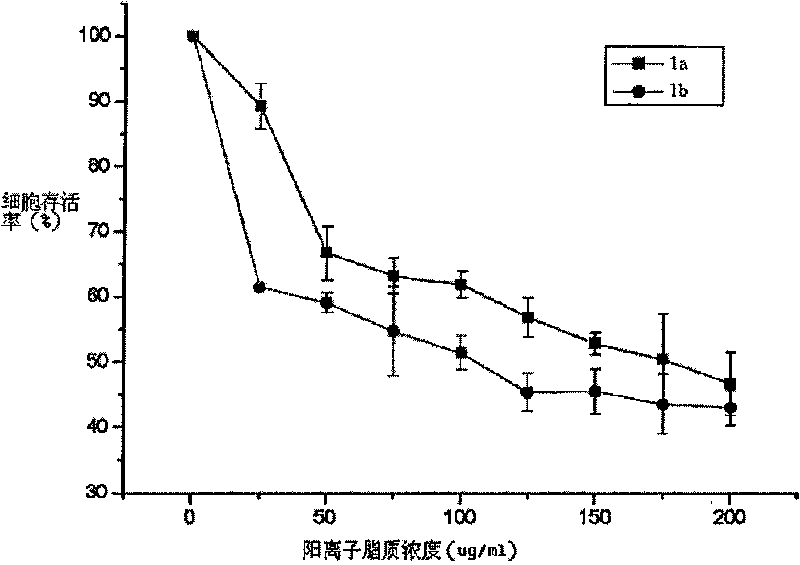
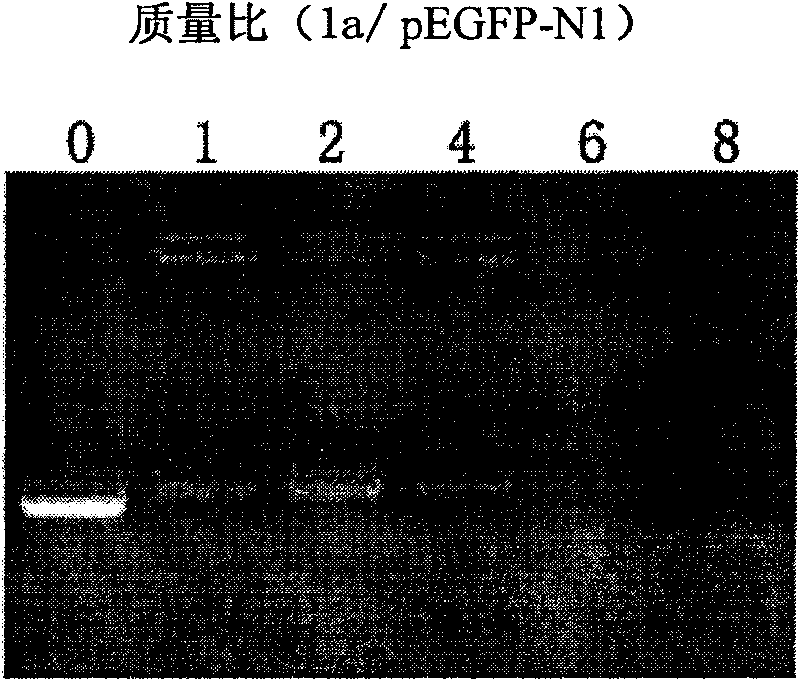
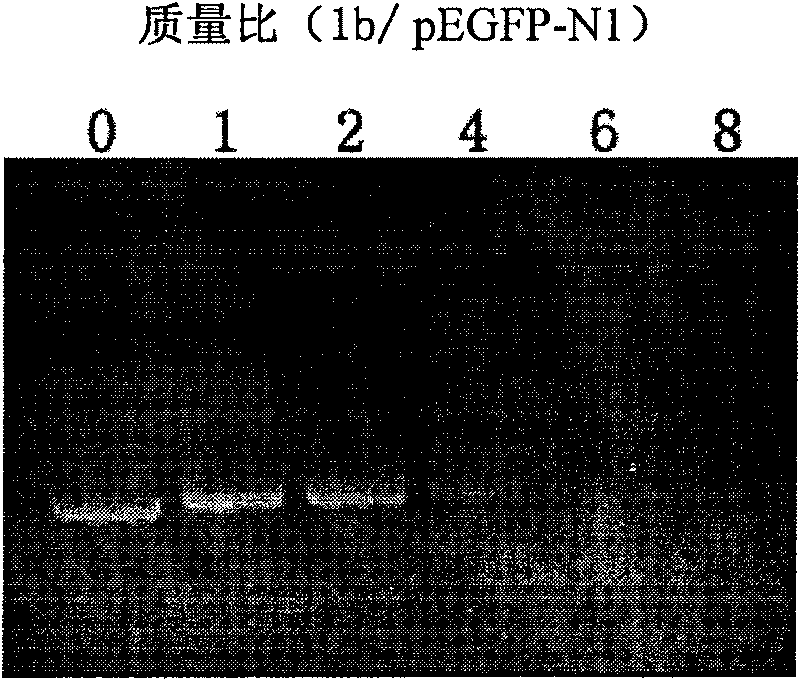
Cation lipid containing imidazolium salt and macrocyclic polyamines, transgenetic vector and method for preparing same
A technology of cationic lipids and transgenic carriers, applied in gene therapy, using carriers to introduce foreign genetic material, pharmaceutical formulations, etc., can solve the problems of high cytotoxicity and achieve the effect of strong encapsulation ability and low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of compound 3a
[0041] Compound 3a is synthesized from compound 2a—cholesteryl chloroacetate, imidazole, and potassium iodide. The structural formula of compound 3a is as follows:
[0042]
[0043] Raw materials include cholesterol chloroacetate, imidazole, potassium iodide and solvent, cholesterol chloroacetate 0.01mol (4.74g), imidazole 0.2mol (13.62g), potassium iodide 0.0005mol (0.08g), solvent from dimethyl sulfoxide and no Composition of water chloroform, dimethyl sulfoxide 10ml, anhydrous chloroform 60ml. Add the above-mentioned solvent, cholesterol chloroacetate, imidazole and potassium iodide into the reaction vessel at room temperature (25°C) and normal pressure, and stir and mix. After the solid matter is completely dissolved, heat to the reflux temperature, and maintain this temperature for reflux reaction. The reaction time is 4 hours. After the reaction is completed, cool to room temperature, then add 2.80 g of anhydrous po...
Embodiment 2
[0044] Embodiment 2: the synthesis of compound 3b
[0045] Compound 3b is synthesized from compound 2b—saponin chloroacetate, imidazole, and potassium iodide. The structural formula of compound 3b is as follows:
[0046]
[0047] Raw material comprises saponin chloroacetate, imidazole, potassium iodide and solvent, saponin chloroacetate 0.013mol (6.30g), imidazole 0.13mol (9.10g), potassium iodide 0.00065mol (0.10g), solvent dimethyl sulfoxide and Composed of anhydrous chloroform, dimethyl sulfoxide 10ml, anhydrous chloroform 40ml. Add the above solvent, saponin chloroacetate, imidazole and potassium iodide into the reaction vessel at room temperature (25°C) and normal pressure and stir and mix. After the solid matter is completely dissolved, heat to reflux temperature and maintain this temperature for reflux Reaction, the reaction time is 6 hours, after the reaction is completed, cool to room temperature, then add 3.00 g of anhydrous potassium carbonate to neutralize the ...
Embodiment 3
[0048] Embodiment 3: the synthesis of compound 5a
[0049] Compound 5a is synthesized from compound 3a and 1-p-bromomethylbenzyl-4,7,10-three (tert-butoxycarbonyl)-1,4,7,10-tetraazacyclododecane, compound 5a The structural formula is as follows:
[0050]
[0051] Raw materials include compound 3a prepared in Example 1, 1-p-bromomethylbenzyl-4,7,10-tri(tert-butoxycarbonyl)-1,4,7,10-tetraazacyclododecane and Solvent anhydrous acetonitrile, compound 3a 2.00mmol (1.04g), 1-p-bromomethylbenzyl-4,7,10-tris(tert-butoxycarbonyl)-1,4,7,10-tetraazacyclo Dodecane 3.30mmol (2.16g), anhydrous acetonitrile 40mL. At room temperature (25°C) and normal pressure, the above-mentioned anhydrous acetonitrile, compound 3a and 1-p-bromomethylbenzyl-4,7,10-tri(tert-butoxycarbonyl)-1,4,7,10 - Tetraazacyclododecane is added to the reaction vessel and stirred and mixed. After the solid matter is completely dissolved, it is heated to reflux temperature, and the temperature is maintained for reflux ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com