Method for preparing chloroaniline by catalysis hydrogenation
A technology for catalytic hydrogenation and chlorinated aniline, which is applied in the preparation of amino compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of decreased catalyst activity, insignificant inhibitory effect, insufficient inhibitory ability, etc., and achieves yield High, obvious inhibition of dechlorination, high reactivity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] In a 250mL autoclave, 2 g of o-chloronitrobenzene, wet Ni-B / SiO 2 Catalyst 2g, ethylenediamine 0.10g, methanol 100mL, hydrogen was introduced 4 times to remove the air, the stirrer was turned on, the stirring speed was 1000r / min, the autoclave was slowly heated to 80°C, and H 2 to 1.0MPa. The reaction time was divided into 15min, 20min and 45min for sampling.
[0010] The reaction results are shown in Table 1.
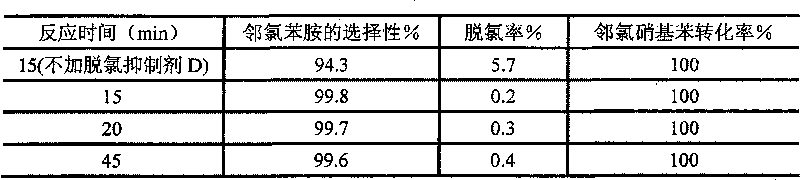
[0011] The impact of table 1 dechlorination inhibitor D on the catalytic reaction
[0012]
[0013] As can be seen from Table 1, adding the dechlorination inhibitor to the catalytic system, the selectivity of generating o-chloroaniline is increased to 99.8% from 94.3% without adding the dechlorination inhibitor. When reacting for 15min, the o-chloronitrobenzene The conversion rate of the catalyst reaches 100%, and the activity is similar to that without adding the dechlorination inhibitor, indicating that the dechlorination inhibitor has no damage to the a...
Embodiment 2
[0015] Catalytic hydrogenation of m-chloronitrobenzene. In a 250mL autoclave, 2 g of m-chloronitrobenzene, wet Ni-B / SiO 2 Catalyst 0.1g, diisopropanolamine 0.10g, methanol 100mL, hydrogen was introduced 4 times to remove air, the stirrer was turned on, the stirring speed was 1000r / min, the autoclave was slowly heated to 363K, and H 2 to 0.5MPa.
[0016] Reaction result: After 15 minutes of reaction, the conversion rate of m-chloronitrobenzene was 100%, the selectivity of m-chloroaniline was 99.9%, and the dechlorination rate was 0.1%.
Embodiment 3
[0018] Catalytic hydrogenation of p-chloronitrobenzene. In the autoclave, 2 g of p-chloronitrobenzene, wet Ni-B / SiO 2 Catalyst 2g, diethanolamine 0.20g, methanol 100mL, hydrogen was passed through 4 times to remove the air, the stirrer was turned on, the stirring speed was 1000r / min, the autoclave was slowly heated to 100°C, and H 2 to 1.0MPa.
[0019] Reaction result: after 15 minutes of reaction, the conversion rate of m-chloronitrobenzene was 100%, the selectivity of p-chloronitrobenzene was 99.9%, and the dechlorination rate was 0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com