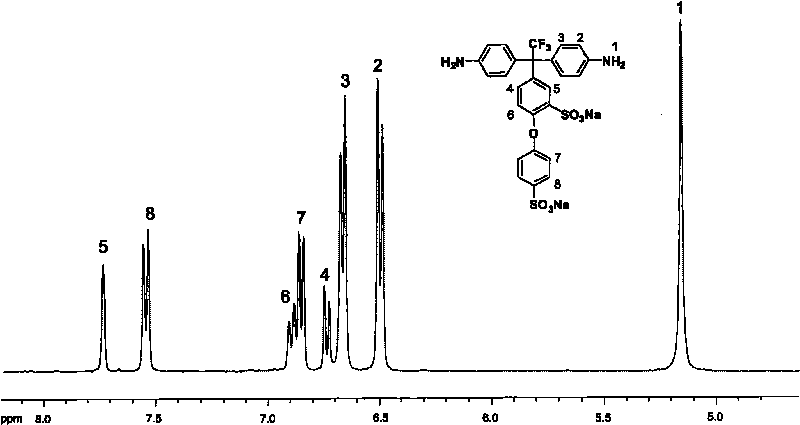
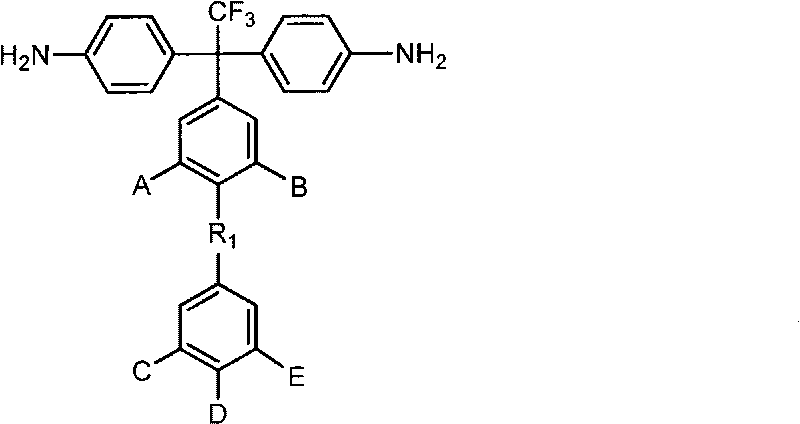
Sulfonated aromatic diamine and sulfonated polyimide resin and preparation methods thereof
A technology of sulfonated polyimide resin and sulfonated aromatic diamine, which is applied in the preparation of sulfonic acid, thioether, organic chemistry, etc., can solve the problem of reducing the thermal stability of thin films, restricting the application of polymers, and making public reports of research results and other problems, to achieve the effects of excellent thermal stability, good proton conductivity, and low methanol permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1, the preparation of sulfonated aromatic diamine
[0055] In a three-neck flask equipped with magnetic stirring, condenser and nitrogen inlet and outlet, add 18.00 grams (0.15 moles) of lithium trifluoroacetate, 2.88 grams (0.12 moles) of magnesium powder and 200 milliliters (2.47 moles) of tetrahydrofuran, under nitrogen protection , stirred and added dropwise 29.13 g (0.12 mol) of 4-bromodiphenyl ether. After the reaction mixture was stirred at room temperature for 4 hours, it was refluxed at 60-70° C. for 4 hours to obtain an off-white turbid solution. After the reaction system was cooled to room temperature, excess magnesium powder and Grignard reagent were reacted with 10% hydrochloric acid aqueous solution. The organic phase was collected and distilled under reduced pressure to obtain 4-phenoxy-α,α,α-trifluoromethylacetophenone liquid.
[0056] 1 H-NMR (400MHz, DMSO-d 6 , ppm): 7.14-7.16 (d, 2H), 7.19-7.21 (d, 2H), 7.29-7.33 (t, 1H), 7.48-7.52 (t, ...
Embodiment 2
[0061] Embodiment 2, the preparation of sulfonated aromatic diamine
[0062] In a three-neck flask equipped with magnetic stirring, condenser and nitrogen inlet and outlet, add 18.00 grams (0.15 moles) of lithium trifluoroacetate, 3.60 grams (0.15 moles) of magnesium powder and 300 milliliters (3.70 moles) of tetrahydrofuran, under nitrogen protection , stirred and added dropwise 31.82 g (0.12 mol) of 4-bromodiphenyl sulfide. After the reaction mixture was stirred at room temperature for 4 hours, it was refluxed at 60-70° C. for 4 hours to obtain an off-white turbid solution. After the reaction system was cooled to room temperature, excess magnesium powder and Grignard reagent were reacted with 15% hydrochloric acid aqueous solution. The organic phase was collected and distilled under reduced pressure to obtain 4-phenylthio-α,α,α-trifluoromethylacetophenone liquid.
[0063] 1 H-NMR (400MHz, DMSO-d 6 , ppm): 7.17-7.19 (t, 1H), 7.23-7.25 (t, 2H), 7.39-7.41 (d, 2H), 7.50-7.52 (...
Embodiment 3
[0068] Embodiment 3, the preparation of sulfonated aromatic diamine
[0069] In the there-necked flask equipped with magnetic stirring, condenser and nitrogen inlet and outlet, add 23.99 grams (0.20 moles) of lithium trifluoroacetate, 4.32 grams (0.18 moles) of magnesium powder and 325 milliliters (4.00 moles) of tetrahydrofuran, under nitrogen protection , stirred and added dropwise 32.50 g (0.15 mol) of 4-chlorobenzophenone. After the reaction mixture was stirred at room temperature for 1 hour, it was refluxed at 60-70° C. for 4 hours to obtain an off-white turbid solution. After the reaction system was cooled to room temperature, excess magnesium powder and Grignard reagent were reacted with 15% hydrochloric acid aqueous solution. The organic phase was collected and distilled under reduced pressure to obtain 4-benzoyl-α,α,α-trifluoromethylacetophenone liquid.
[0070] 1 H-NMR (400MHz, DMSO-d 6 , ppm): 7.50-7.55 (t, 2H), 7.60-7.64 (t, 1H), 7.76-7.78 (d, 2H), 7.87-7.89 (d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com