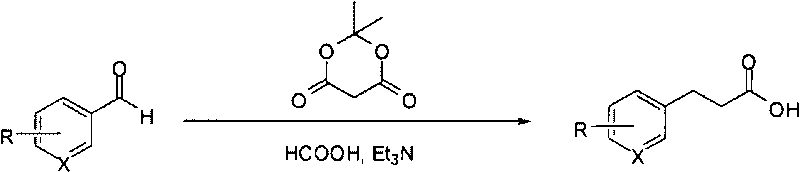
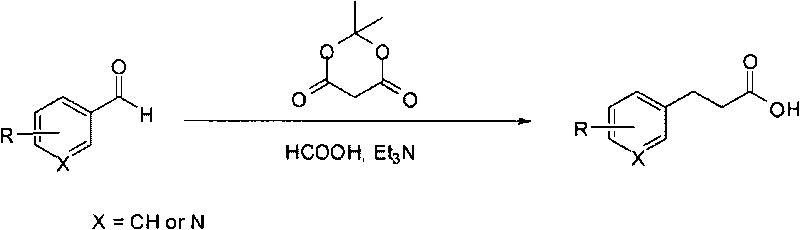
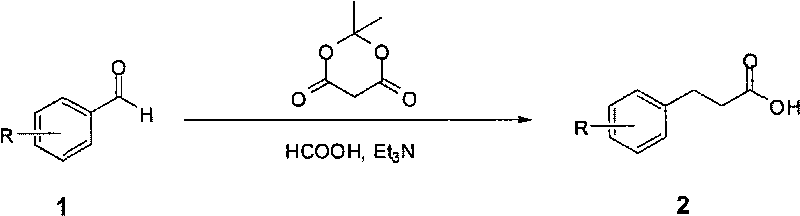
Method for fast synthesizing 3-substituted aromatic acid
A technique for the synthesis of aryl propionic acid, which is applied in chemical instruments and methods, preparation of carboxylic acid esters, preparation of organic compounds, etc., can solve problems such as difficult separation and purification, limited applicability, complicated post-treatment, etc., and achieves synthesis The effect of simple process, high cost of improvement, and simple purification and separation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 7
[0011]
[0012] Table 1: Synthesis of the corresponding 3-substituted phenylpropionic acid from substituted benzaldehyde
[0013]
[0014] a: separation yield b: HPLC purity
[0015] Typical reaction steps: add 2-methoxymethylbenzaldehyde (2g, 13.3mmol) and dimethylidene malonate (1.92g, 13.3mmol) to dry 5mL formic acid and 13mL triethylamine, reflux Stir for 10 hours; cool the reaction solution in an ice bath, add 20 mL of water, and then add 1M NaOH solution dropwise to adjust pH=9-10. The resulting solution was washed with ethyl acetate (2×20mL), the separated aqueous phase was added dropwise with 6M HCl solution to adjust the pH=2-4, extracted with ethyl acetate (2×50mL), the ethyl acetate phases were combined, and anhydrous sulfuric acid was added Sodium is dry. After filtration, the filtrate was concentrated under reduced pressure to obtain 3-(2'-methoxymethylphenyl)propionic acid.
[0016] 2a. Proton nuclear magnetic resonance spectroscopy 1 H NMR(CDCl 3 , 400MHz), δppm: 7....
Embodiment 8 to 11
[0030]
[0031] Table 2: Synthesis of the corresponding 3-substituted aromatic propionic acid from substituted aromatic formaldehyde
[0032]
[0033] a: separation yield b: HPLC purity
[0034] 4a. Proton nuclear magnetic resonance spectroscopy 1 H NMR(CDCl 3 , 400MHz), δppm: 7.96(d, J=8.0Hz, 1H), 7.55(t, J=7.2Hz, 1H), 7.43-7.38(m, 2H), 3.24(t, J=7.6Hz, 2H) , 2.80(t, J=7.6Hz, 2H)
[0035] HPLC purity: 98%; yield: 85%
[0036] 4b. Proton nuclear magnetic resonance spectroscopy 1 H NMR(DMSO-D 6 , 400MHz), δppm: 12.15 (brs, 1H), 7.42~7.33 (m, 1H), 7.23-7.19 (m, 2H), 2.79 (t, J = 7.6 Hz, 2H), 2.52 (t, J = 7.6 Hz, 2H)
[0037] HPLC purity: 97%; yield: 84%
[0038] 4c. Proton nuclear magnetic resonance spectroscopy 1 H NMR(CD 3 OD, 400MHz), δppm: 8.27-8.25 (dd, J 1 = 6.0 Hz, J 2 =1.2Hz, 1H), 8.19-8.17 (dd, J 1 =8.8Hz, J 2 =1.2Hz, 1H), 7.92-7.88 (dd, J 1 =8.8Hz, J 2 =5.6Hz, 1H), 4.08(s, 3H), 3.26(t, J=7.2Hz, 2H), 2.83(t, J=7.2Hz, 2H)
[0039] HPLC purity: 93%; yield: 72%
[0040] 4d. Proton nu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com