Synthesis method of etretinate ether
A compound, selected technology, applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of unsafe operation, harsh reaction conditions, long reaction time, etc. mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
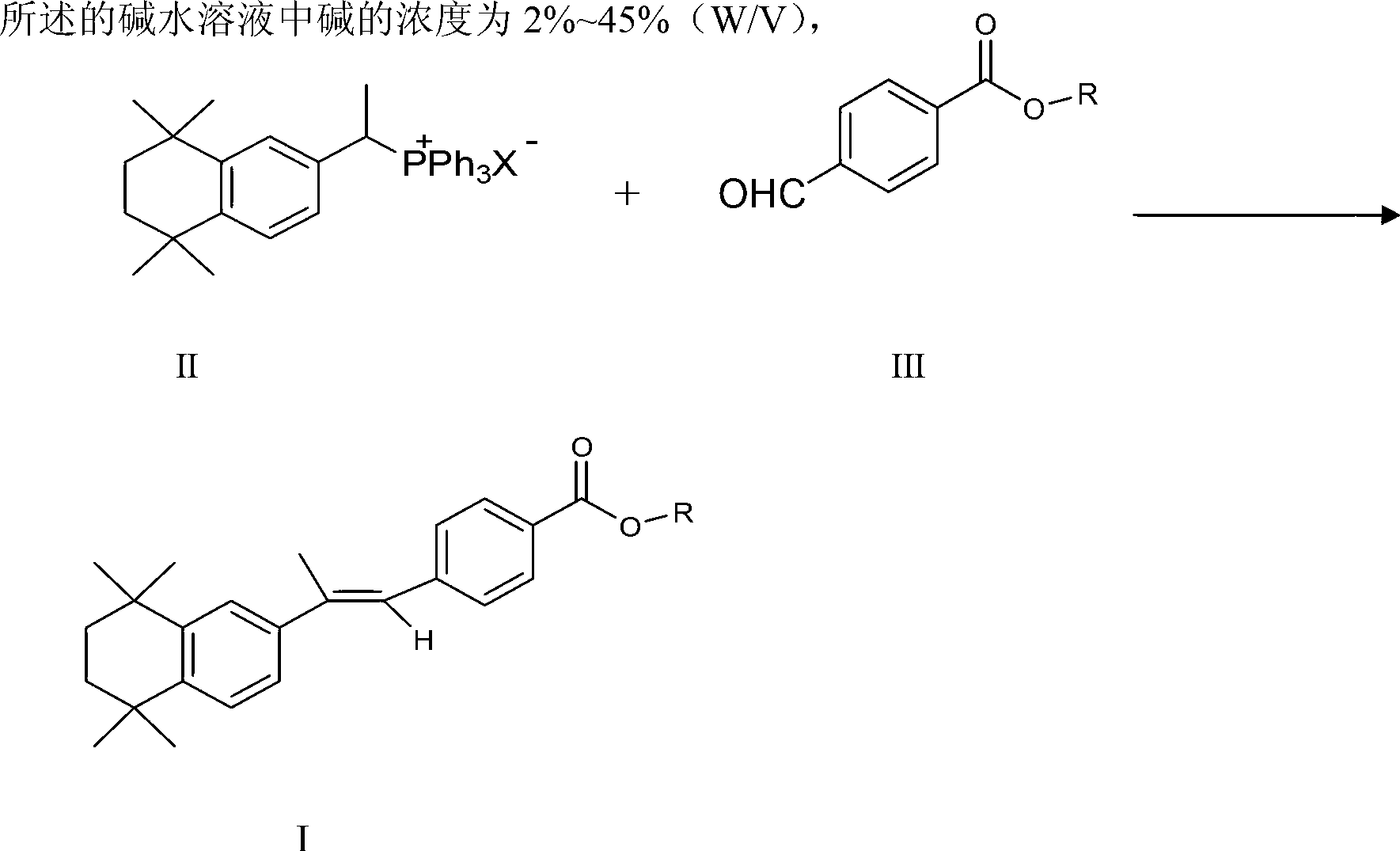
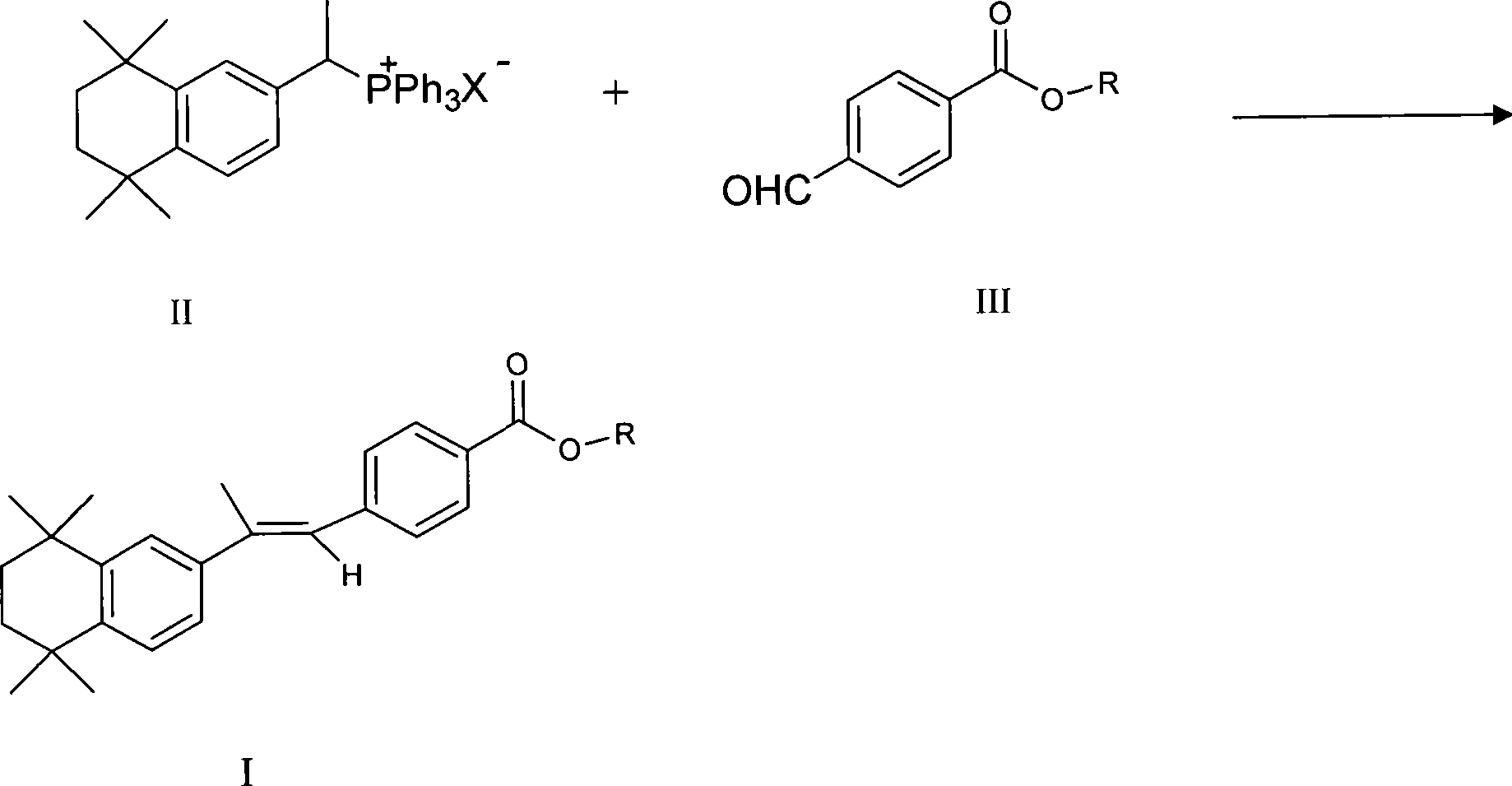
[0033] Example 1 Synthesis of 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)propenyl]benzoate (one)
[0034] 10g [1-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)-ethyl]-triphenylphosphonium bromide, 4.0g p-methoxy Add carbonylbenzaldehyde to 200ml of dichloromethane, then add 200ml of 2% NaOH aqueous solution, stir at 40°C for two-phase reaction for about 1h, TLC (developing agent: methanol: chloroform = 1:5) to monitor the completion of the reaction of raw materials After the reaction, the organic layer was separated, and the organic solvent was removed after the organic layer was dried. Add 60ml of methanol to the residue, the precipitated solid is the crude product; heat dissolve the crude product in 20ml of ethyl acetate, add 60ml of ethanol, and precipitate needle-like crystals to obtain the title compound; the yield is 61.17%, and the content (HPLC) is 99.47%.
Embodiment 2
[0035] Example 2 Synthesis of 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)propenyl]benzoic acid methyl ester (two)
[0036] 10g [1-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)-ethyl]-triphenylphosphonium bromide, 5.8g p-methoxy Add carbonylbenzaldehyde to 300ml of dichloromethane, then add 12ml of 33% NaOH aqueous solution, stir at 40°C for two-phase reaction for about 5 hours, TLC (developing agent: methanol: chloroform = 1:5) to monitor the completion of the raw material reaction After the reaction, the organic layer was separated, and the organic solvent was removed after the organic layer was dried. 60ml of methanol was added to the residue, and the precipitated solid was the crude product. The crude product was dissolved in 20ml of ethyl acetate, and 60ml of ethanol was added to precipitate needle-like crystals to obtain the title compound with a yield of 79.93% and a content (HPLC) of 99.20%.
Embodiment 3
[0037] Example 3 Synthesis of 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)propenyl]benzoic acid methyl ester (three)
[0038] 9.3g [1-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)-ethyl]-triphenylphosphine chloride, 4.8g p-methyl Add oxycarbonylbenzaldehyde to 250ml of carbon tetrachloride, then add 10ml of 45% NaOH aqueous solution, stir at 70°C for two-phase reaction for about 1h, TLC (developing agent: methanol: chloroform = 1:5) to monitor the raw materials After the reaction is completed, the organic layer is separated after the reaction, and the organic solvent is removed after the organic layer is dried. 60ml of methanol was added to the residue, and a solid was precipitated to obtain a crude product. The crude product was dissolved in 20ml of ethyl acetate, and 60ml of ethanol was added to precipitate needle-like crystals to obtain the title compound. The yield was 71.24%, and the content (HPLC) was 99.30%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com