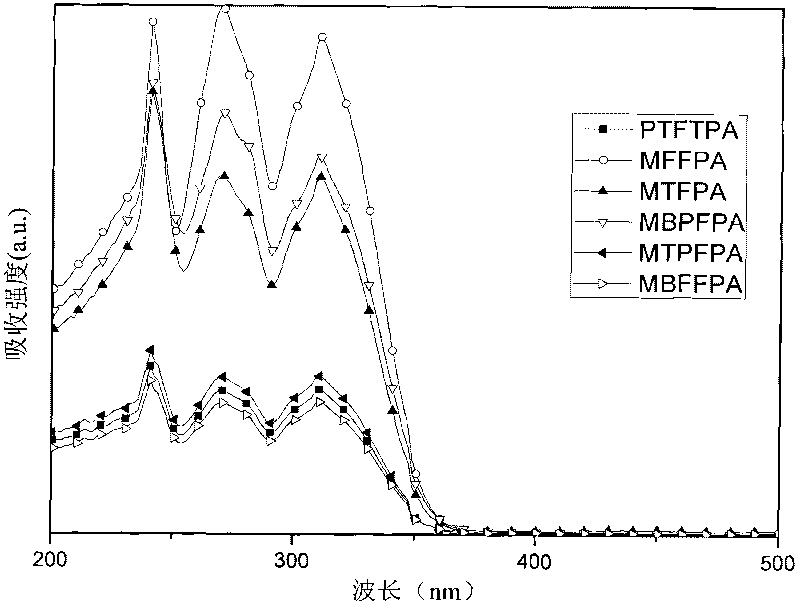
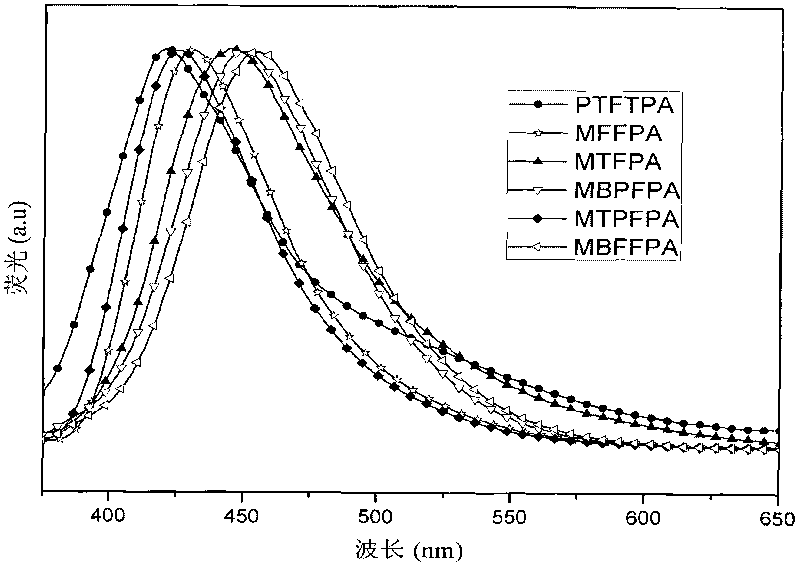
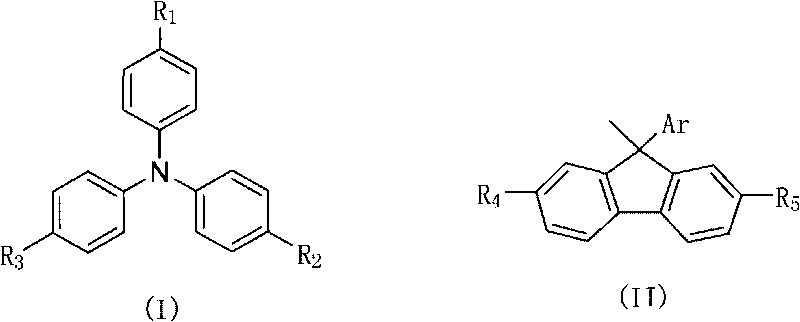
Non-conjugated derivative material with fluorine-triphenylamine structure and synthesis method thereof
A synthesis method and technology of triphenylamine, which are applied in the field of synthesis of non-conjugated derivative materials, can solve the problems affecting the saturated color purity, efficiency and stability of devices, difficulty in injection of holes and electrons, mismatch of cathode work functions, etc. Achieve the effects of improving thermal stability and film-forming properties, cheap raw materials, and improving injection and transport capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Step 1: Synthesis of Compound 9-p-tolyl-9H-fluoren-9-ol, PTF
[0035] p-Bromotoluene (40mmol, 6.8g) was dissolved in 15ml of anhydrous ether, magnesium powder (40mmol, 0.96g) and a little iodine to react to generate Grignard reagent, which was placed in an ice bath, and fluorenone (20mmol 3.6g) Dissolved in 20ml of anhydrous ether solution, added dropwise to Grignard reagent to react for 3h. Add saturated ammonium chloride solution dropwise to quench the reaction, add chloroform to extract, then use petroleum ether / ethyl acetate=20 / 1 as the eluent silica gel chromatography to obtain the crude product, and then pass through diethyl ether / n-hexane (5 / 1) Recrystallization obtained 4.3 g of the target product PTF with relatively high purity, and the yield was 79%.
[0036] 1 H NMR (CDCl 3500MHz) δ7.64(d, J=7.5Hz 2H), 7.31-7.29(m, 4H), 7.06-7.26(m, 4H), 7.05(d, J=8Hz 2H), 2.48(s, 1H), 2.28(s, 3H)
[0037] 12 C NMR (CDCl 3 500MHz) δ150.6, 140.3, 139.6, 136...
Embodiment 2
[0043]
[0044] Step 1: Synthesis of 9-(4-fluorophenyl)-9H-fluoren-9-ol, FPF
[0045] According to the operation of step 1 in Example 1, p-bromofluorobenzene (40mmol, 7g), magnesium powder (4mmol, 0.96g), and fluorenone (10mmol, 1.8g) gave the product FPF (2.42g, 87.6%).
[0046] 1 H NMR (CDCl 3 500MHz) δ7.83-7.82(m, 2H), 7.39-7.35(m, 2H), 7.29-7.25(m, 6H), 7.08-7.04(m, 2H), 6.40(s, 1H)
[0047] 12 C NMR (CDCl 3 500MHz) δ162.1, 160.1, 151.1, 141.3, 141.2, 139.1, 128.6, 128.1127.1, 124.6, 120.1, 114.8, 114.6, 82.2
[0048] Step 2: Compound 4-methyl-N, N-bis(4-(9-(4-fluor-ophenyl)-9H-fluoren-9-yl)-phenyl-)aniline, synthesized by MFFPA
[0049] According to the operation of step 2 in Example 1, 4-methyltriphenylamine (1 mmol, 0.26 g) and FPF (2.2 mmol, 0.61 g) were reacted for 3 h to obtain the target product MFFPA (0.72 g, 92.9%).
[0050] 1 H NMR (CDCl 3 500MHz) δ7.73(d, J=8.0, 4H), 7.36-7.32(m, 8H), 7.26-7.23(m, 4H), 7.15(dd, J=8.5, 4H), 7.02-6.93(m, 8H), 6.89-6...
Embodiment 3
[0053]
[0054] Step 1: Synthesis of Compound 9-(4-tert-butylphenyl)-9H-fluoren-9-ol, TBPF)
[0055] According to the operation of step 1 in Example 1, p-tert-butylbromobenzene (20mmol, 4.3g), magnesium powder (20mmol, 0.48g), fluorenone (10mmol, 1.8g) obtained product TBPF (2.91g, 92.7%) .
[0056] 1 H NMR (CDCl 3 500MHz ppm) δ7.63(dd, J=7.0, 2H), 7.34-7.31(m, 4H), 7.29-7.24(m, 4H), 7.23-7.20(m, 4H), 2.45(s, 1H), 1.26(s, 9H).
[0057] 12 C NMR (CDCl 3 500MHz ppm) δ150.4, 149.3, 140.1, 139.5, 128.9, 128.3, 125.1, 125.0, 124.8, 120.0, 83.5, 34.4, 31.3.
[0058] Step 2: Synthesis of compound 4-methyl-N, N-bis(4-(9-(4-tert-butylphenyl)-9H-fluoren-9-yl)phenyl)aniline, MTFPA
[0059] 4-Methyltriphenylamine (1mmol, 0.26g), TBPF (2.2mmol 0.69g) were dissolved in 80ml of dichloromethane, and then 20ml of dichloromethane dissolved with trifluoromethanesulfonic acid (5.5mol 0.82g) was slowly Added, reacted at room temperature for 1 h, washed three times with saturated sodiu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com