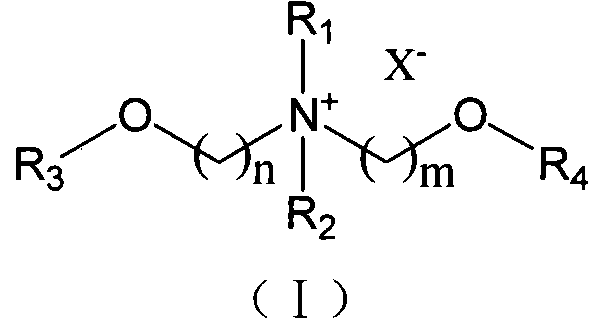
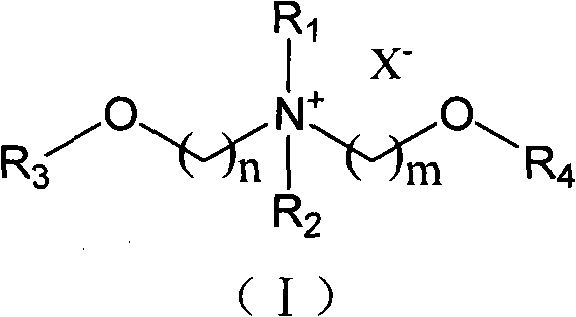
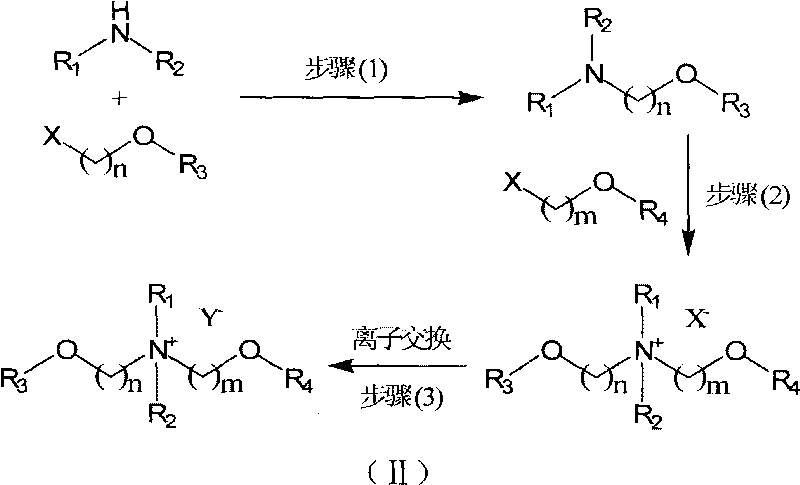
Ionic liquid taking diether quaternary ammonium as cation and synthetic method thereof
A technology of ionic liquids and cations, applied in chemical instruments and methods, preparation of amino hydroxyl compounds, preparation of organic compounds, etc., to achieve the effects of low viscosity, low curing temperature, and high conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: N, N-dimethyl N-(2-methoxyethyl) amine (N 11,1O2 )Synthesis
[0038] 278g (2.0 moles) of 2-bromoethyl methyl ether was added dropwise (12 hours) to 684g 33% aqueous dimethylamine solution (containing 5.0 moles of dimethylamine) at 30°C. After the addition was complete, the reaction temperature was raised to 40°C, stop after 12h. Add 2.0 moles of NaOH to neutralize the generated acid, and add an appropriate amount of NaCl to make it a saturated solution. Atmospheric pressure distillation, collecting fractions at 80-90°C, successively using CaCl 2 Dry with KOH and rinse with (3×100ml) anhydrous ether when filtering. Atmospheric distillation again, collecting fractions at 96-98°C to obtain the product N,N-dimethyl-N-(2-methoxyethyl)amine (N 11,1O2 ) 129g, yield 63%.
Embodiment 2
[0039] Embodiment 2: N, N-dimethyl-N-methoxymethyl-N-(2-methoxyethyl) quaternary ammonium chloride salt ([N 11,1O1,1O2 ] + [Cl] - )Synthesis
[0040] Under ice-bath conditions, slowly drop 0.80 moles (60.8ml) of chloromethyl methyl ether into the mixture containing 0.80 moles (82.5g) of N 11,1O2 and 100ml of dichloromethane, and then reacted at 30°C for 4 hours (the reaction system must be connected to a drying tube to isolate the moisture in the air). The solvent was removed under reduced pressure at 50°C to obtain a light yellow liquid [N 11,1O1,1O2 ] + [Cl] - 147 g, >99% yield.
Embodiment 3
[0041] Embodiment 3: N, N-dimethyl-N, N-two (2-methoxyethyl) quaternary ammonium chloride salt ([N 11,1O2,1O2 ] + [Cl] - )Synthesis
[0042] 0.80 moles (82.5g) of N 11,1O2 Add 0.8 moles (76g) of 2-chloroethyl methyl ether and 80ml of absolute ethanol into a sealed reaction tank, and react at 120°C for 48 hours. After taking it out, molecular impurities were removed under reduced pressure at 70°C to obtain a white solid [N 11,1O2,1O2 ] + [Cl] - 168 g, yield: 85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com