Method for labeling antibody by fluorophore generated by combination of iridium coordination compound and histidine
A biomarker and coordination compound technology, applied in instruments, analytical materials, peptides, etc., can solve the problems of fluorescent labeling with huge structures and inability to carry out polypeptides and proteins, and achieve stable labeling products, good photostability, and stable properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
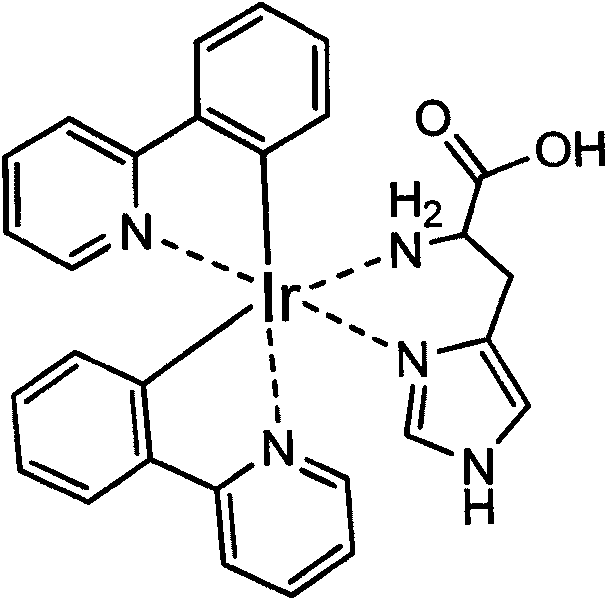
[0030] Implementation Example 1: Complexation reaction of iridium coordination compound with four compounds such as histidine
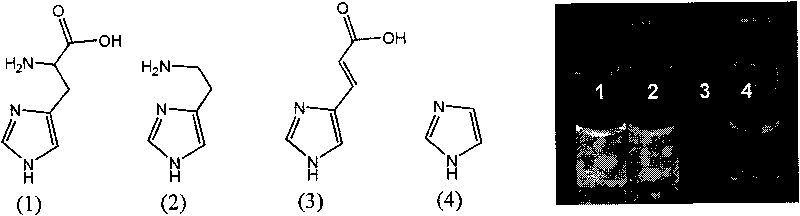
[0031] [(mppy) 2 Ir(CH 3 EN) 2When ](OTf) reacts with four compounds containing imidazole groups such as histidine (L-histidine, histamine, urocanic acid and imidazole), [(mppy) 2 Ir(CH 3 EN) 2 ](OTf) and the concentration of the four substances are all 50 μM, and the reaction is carried out in PBS (pH 7.4). After mixing in the test tube, the reaction is allowed to stand at room temperature for about 10 minutes. figure 2 The chemical structures of the four substances are shown respectively, as well as the experimental results after the respective reactions are completed. Under the irradiation of ultraviolet light (365nm), L-histidine and histamine are the same as [(mppy) 2 Ir(CH 3 EN) 2 ](OTf) can produce fluorescence, while the other two compounds can not produce fluorescence. The result speculated from this is that -NH in histidine 2 Part...
Embodiment 2
[0032] Implementation Example 2: Complexation reaction of iridium coordination compound with histidine-containing polypeptide
[0033] [(mppy) 2 Ir(CH 3 EN) 2 ](OTf) and two polypeptide chains (one of which contains histidine residues: KQEA H GSTA, another as a control, does not contain histidine residues: KQEASGSTA) when reacting, [(mppy) 2 Ir(CH 3 EN) 2 ](OTf) and the concentration of the polypeptide are both 50 μM, and the reaction is carried out in PBS (pH 7.4). After the reactants were mixed in the test tube, they were allowed to stand at room temperature for about 10 minutes. After the two polypeptides reacted with the iridium coordination compound, the fluorescence ( Figure 6 ). Figure 7 The fluorescence emission spectrum under 328nm excitation of the histidine-containing polypeptide combined with the iridium coordination compound is shown. Since the peptide [1] does not contain a histidine residue and the sequence of the polypeptide [2] contains a histidine ...
Embodiment 3
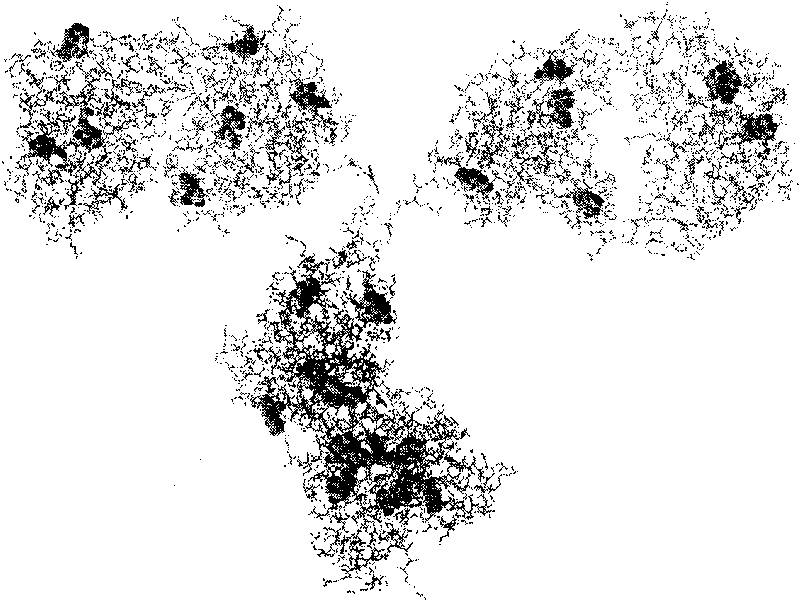
[0034] Implementation Example 3: Complexation reaction of iridium coordination compound with rabbit anti-sheep immunoglobulin IgG
[0035] [(mppy) 2 Ir(CH 3 EN) 2 ] (OTf) concentration was 50 μM, the concentration of rabbit anti-goat immunoglobulin IgG was 1 mg / mL, and the reaction was carried out in PBS (pH 7.4). After the above substances or the control group were mixed in the test tube, they were allowed to stand at room temperature for about 10 minutes to react. Figure 9 After showing that the reaction is over, under the irradiation of ultraviolet light (365nm) [(mppy) 2 Ir(CH 3 EN) 2 ](OTf) Fluorescence generated after binding rabbit anti-goat IgG. Figure 10 Fluorescence emission spectra of IgG-bound iridium-emitting compounds under excitation at 328 nm are shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com