Method for regenerating and recovering sulfuric acid from waste acid
A waste acid and sulfuric acid technology, applied in the direction of sulfur trioxide/sulfuric acid, chemical instruments and methods, water pollutants, etc., can solve the problems of no large-scale production and application, no sulfuric acid regeneration, and affecting the quality of sulfuric acid, etc., to achieve Huge environmental and social benefits, reduced water consumption, and pollution avoidance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
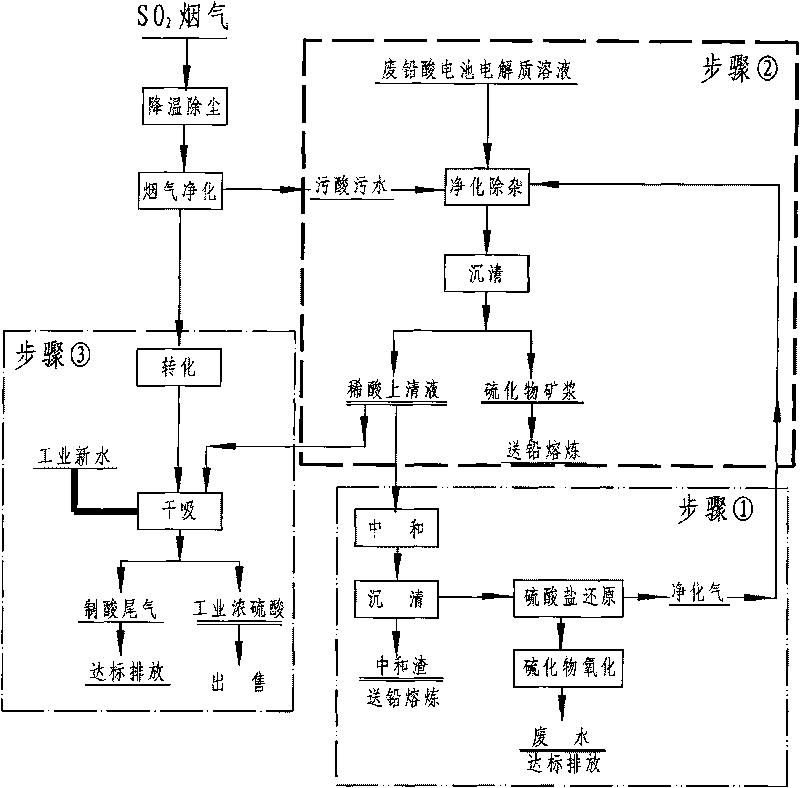
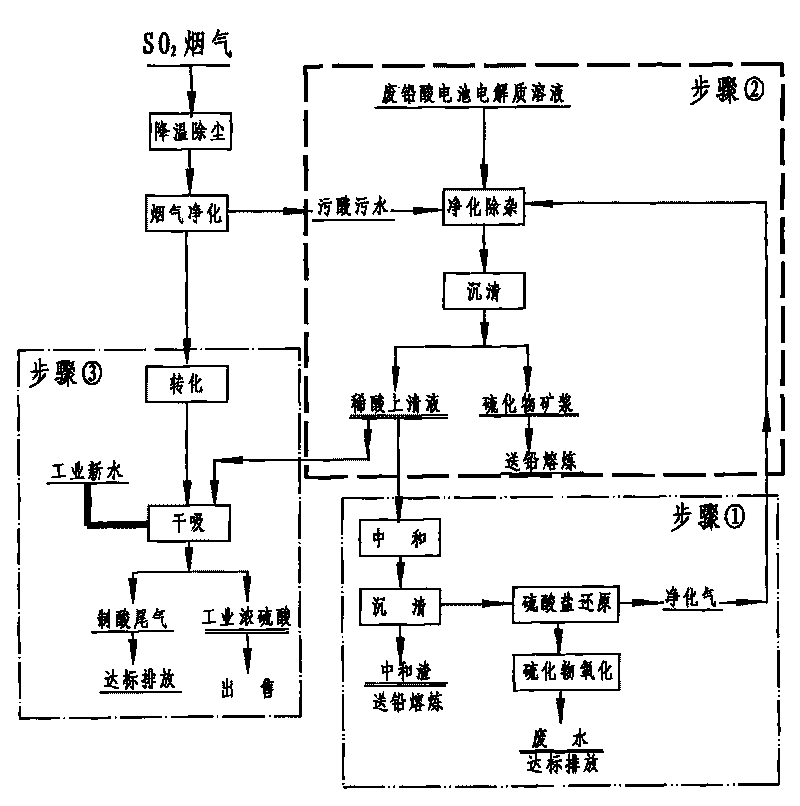
[0025] Embodiment 1: as figure 1 As shown, the SO 2 A large amount of dirty acid solution is produced during the purification process, except for 10% H 2 SO 4 In addition, it also contains harmful impurity elements such as lead, arsenic, antimony, and cadmium. When studying how to effectively regenerate and recover sulfuric acid in these waste sewage acid solutions, a method for regenerating sulfuric acid from waste sewage acid is adopted, which is characterized in that it includes Follow the steps below:
[0026] Step 1: The purchased H 2 S, CO 2 , N 2 The three gases contain H 2 S 25 liters, CO 2 200 liters, N 2 775 liters are mixed to make 1000 liters of purified gas;
[0027] Step 2: Pass the purified gas to the flow rate of 10-20m at a rate of 400-600L / h 3 / h, the stirring speed is in the waste sewage acid solution of 300-400rpm, make the heavy metal impurity in the waste sewage acid and S 2+ The reaction generates sulfides, and the purification and impurity r...
Embodiment 2
[0030] Embodiment 2: as figure 1 As shown, the secondary lead enterprises with flue gas acid production produce a small amount of electrolyte solution containing heavy metals when dismantling waste batteries. In order to effectively recover the sulfuric acid in it, a method of regenerating sulfuric acid from waste acid is adopted. It consists of the following steps:
[0031] Step 1: The purchased H 2 S, CO 2 , N 2 The three gases contain H 2 S 25 liters, CO 2 225 liters, N 2 250 liters of volume percentage are mixed to make 500 liters of purified gas;
[0032] Step 2: intermittently feed the purified gas at a rate of 400-600L / h to a volume of 20m 3 , stirring speed is 300-400rpm, filled with 15m 3 In the reaction kettle of the waste sewage acid solution, the heavy metal impurities in the waste sewage acid solution and S 2+ The reaction produces insoluble sulfides and precipitates, and the purification and impurity removal process of passing the purification gas into t...
Embodiment 3
[0035] Embodiment 3: as figure 1 As shown, there is an electrolyte solution containing a large amount of heavy metals in the waste batteries after use, and SO 2 During the flue gas purification process, a large amount of 10% H 2 SO 4 waste acid solution, for effectively regenerating and reclaiming the sulfuric acid in these waste acid solutions, adopting a method for regenerating and reclaiming sulfuric acid from waste acid, characterized in that: comprising the steps:
[0036] Step 1: First add the pure sulfate solution prepared from purchased sulfate or the sulfate solution produced after neutralization and sedimentation in the process of waste acid treatment into the sulfate biological reduction anaerobic reactor, and continuously Continuous preparation containing H 2 S 2.5-5%, CO 2 20-45%, N 2 50-77.5% purified gas;
[0037]Step 2: Pass the purified gas to the flow rate of 10-20m at a rate of 400-600L / h 3 / h, the stirring speed is in the waste sewage acid solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com