Method for preparing monocyte type stem cell and application
A mononuclear cell, stem cell technology, applied in the field of cell separation, to reduce the risk and spread of disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Isolation and cultivation of monocyte-type stem cells from peripheral blood.
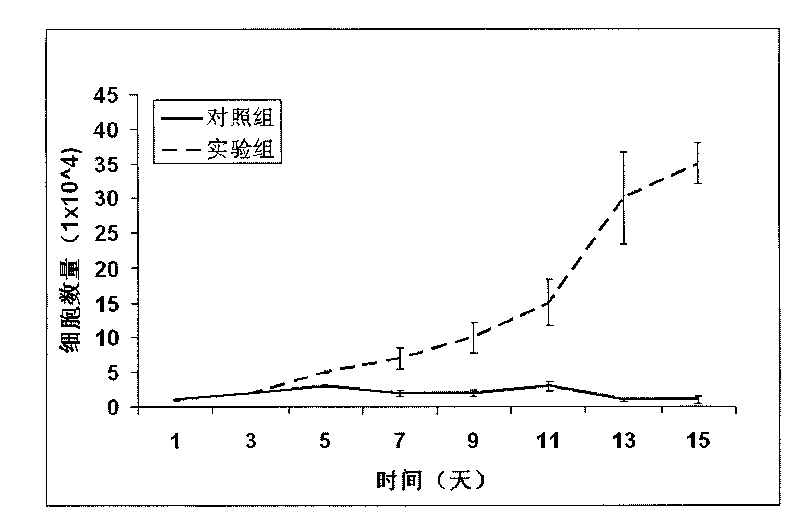
[0041] 200 ml of peripheral blood was extracted, diluted with RPMI 1640 to an equal volume, added with Ficoll-Hypaque, and then centrifuged (550 g) in a Beckmann centrifuge for 30 minutes at 4°C. The harvested buffy coat was washed 2-3 times with RPMI 1640. These cells can be stored immediately in liquid nitrogen or cultured to isolate monocytes. According to the concentration of 2 million cells / milliliter, after adding the culture medium to dilute, transfer the cells to the culture dish for 8-12 hours (37°C, 8% CO2), remove the floating cells, wash 5 times with RPMI1640 medium. Add 5-10 ml of RPMI 1640 medium containing 10% calf serum, and forcefully wash the cells attached to the culture dish to obtain 90-95% pure monocytes. 99% pure monocytes can be further obtained by antibody-binding magnetic bead method. When monocytes were cultured for 5 days by adding 50 ng / ml of M-CSF, 1...
Embodiment 2
[0043] Example 2 Differentiation of macrophages, T lymphocytes and NK cells
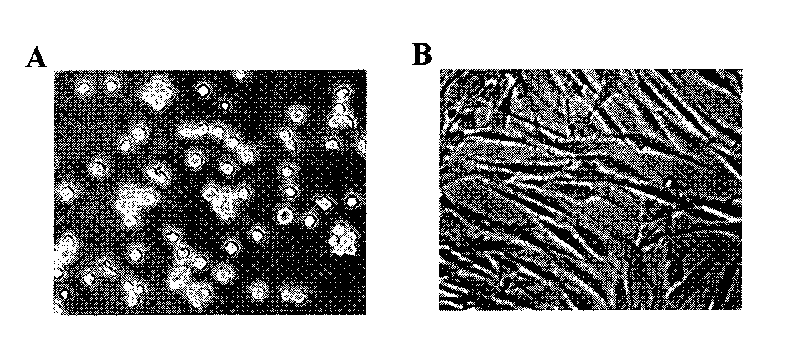
[0044] In order to confirm that monocyte-type stem cells have the ability to differentiate into T lymphocytes, monocytes treated with 50 ng / ml M-CSF, 1000 activity units / ml LIF, and 20 ng / ml IL-6 for 14 days Add 5 ng / ml lipopolysaccharide (macrophage activating factor) for two days. The monocyte-type stem cells thus treated are converted into macrophages. This shift was confirmed by the following experiments, morphological features, increases in HLA-DR, HLA-DQ, immunostaining for IL-10 and TNF-a ( image 3 , Figure 4 ).
[0045] In order to confirm that monocyte-type stem cells have the ability to differentiate into T lymphocytes, monocytes treated with 50 ng / ml M-CSF, 1000 activity units / ml LIF, and 20 ng / ml IL-6 for 14 days Add 1200 activity units / ml of IL-2 (T lymphocyte activating factor) and culture for 4 days. The monocyte-type stem cells thus treated are transformed into T lymphocytes. ...
Embodiment 3
[0047] Example 3 Epithelial cell differentiation
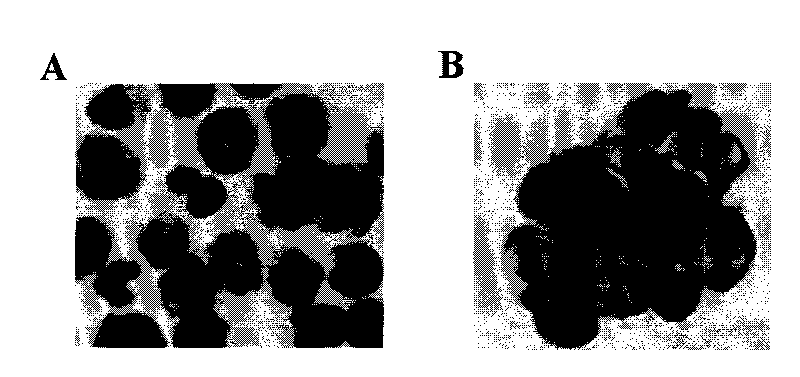
[0048]In order to confirm that monocyte-type stem cells have the ability to differentiate into epithelial cells, monocytes treated with 50 ng / ml M-CSF, 1000 activity units / ml LIF, and 20 ng / ml IL-6 for 14 days were added 100 ng / ml epidermal growth factor (EGF), cultured for 4 days. 75% of the monocyte-type stem cells thus treated showed an epithelial morphology, 71 ± 4% of the cells showed an epithelial morphology after immunostaining, and 68 ± 5% of the cells were positive for E-cadherin, both proteins of which are epithelial marker protein. In the control group, only 4±1% pan-keratins were positive, and 3±2% E-cadherin was positive ( Figure 6 ). Therefore, the monocyte-type stem cells of the present invention can be induced to differentiate into other types of cells other than blood cells. Suitable differentiation-inducing doses can be determined by those of ordinary skill in the art using known routine techniques.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com