Oseltamivir dry syrup and preparation method thereof
A technology of oseltamivir and oseltamivir phosphate, which is applied in antiviral agents, pharmaceutical formulas, medical preparations of non-active ingredients, etc., can solve the problems of impurities without considering stability, and achieve uniform appearance and convenient administration , the effect of uniform content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] 4. Preparation of adhesive (50% ethanol): Weigh the prescribed amount of ethanol:purified water=1:1 to prepare an adhesive for later use.
[0035] 5. Mixing and granulation: put the mixed material in a wet mixing granulator, add ethanol to make a soft material with a binder according to the weight of the material at 40-50% by weight, the specific conditions are as follows: stirring parameters 500rpm, shearing 1000rpm for granulation The time is 3 minutes. The prepared soft material is placed in an extrusion spheronizer with a screen size of 0.6.
[0036] 6. Drying: Use a fluidized bed to dry the granulation, the drying temperature is 30-40°C, and the drying moisture is controlled below 0.5%.
[0037] 7. Packing: Calculated based on the product content of 30 mg / g, each pack should contain 0.9 g of oseltamivir phosphate, and the filling volume is about 30 g.
Embodiment 1
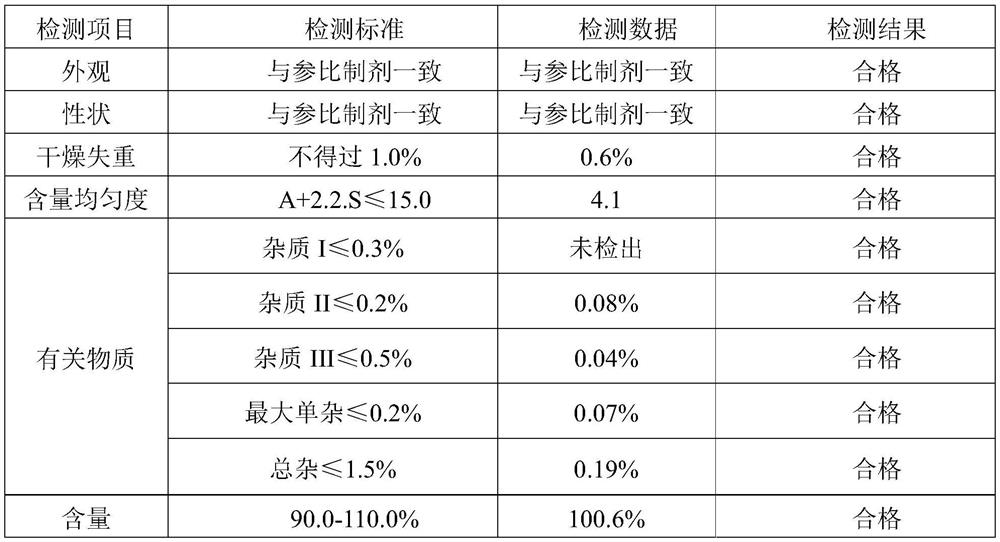
[0040] Embodiment 1 Oseltamivir phosphate dry syrup detection result is as follows:
[0041]
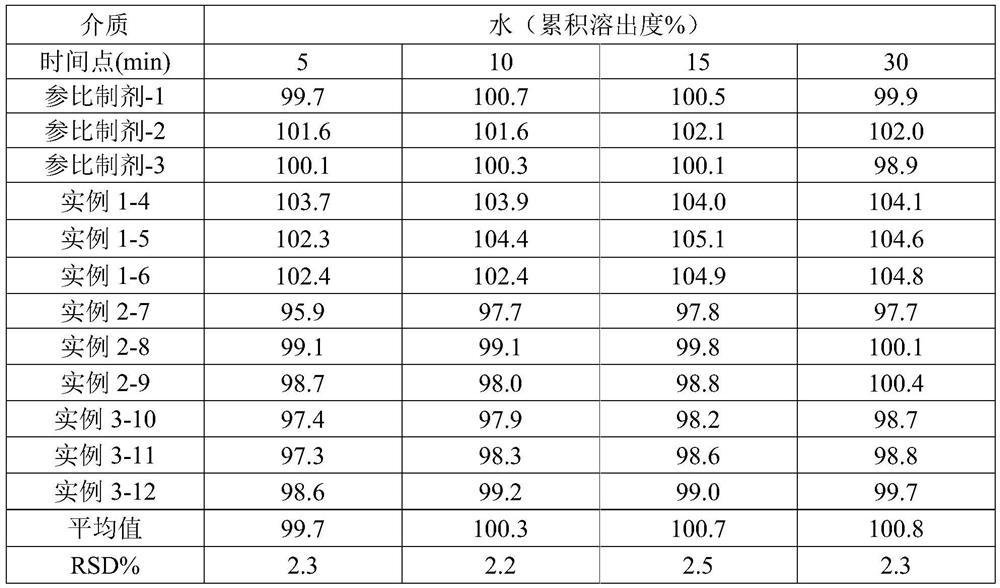
[0042] Cumulative dissolution test results
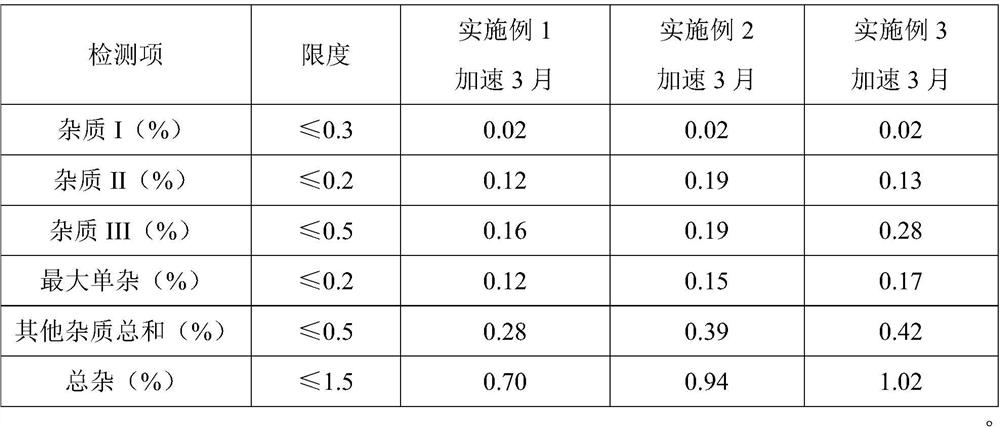
[0043] In order to observe the performance of the reference preparation more intuitively, put the product prepared according to the above process into a stability box, and place it at a high temperature of 60°C and a high humidity of 90% for 10 days to evaluate the formulation differences of the samples under different conditions. The result is shown in table 3, as can be seen, the stability of embodiment 1, 2 is better, and the stability of embodiment 3 is poor, all does not exceed the item of prescribed limit, in total miscellaneous items, the impurity amount of implementation case 1 It is obviously less than the implementation case 2, and has the advantage of being more stable. It shows that the selection of sorbitol can significantly improve the stability of the preparation, and the preparation of the present invention has similar ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com