Preparation method of magnesium oxide by using bittern and carbonate
A carbonate and magnesium oxide technology, applied in magnesium oxide and other directions, can solve the problems of high free ammonium concentration, poor operating environment, environmental pollution, etc., and achieve the effects of low energy consumption, easy operation, easy manufacturing and processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
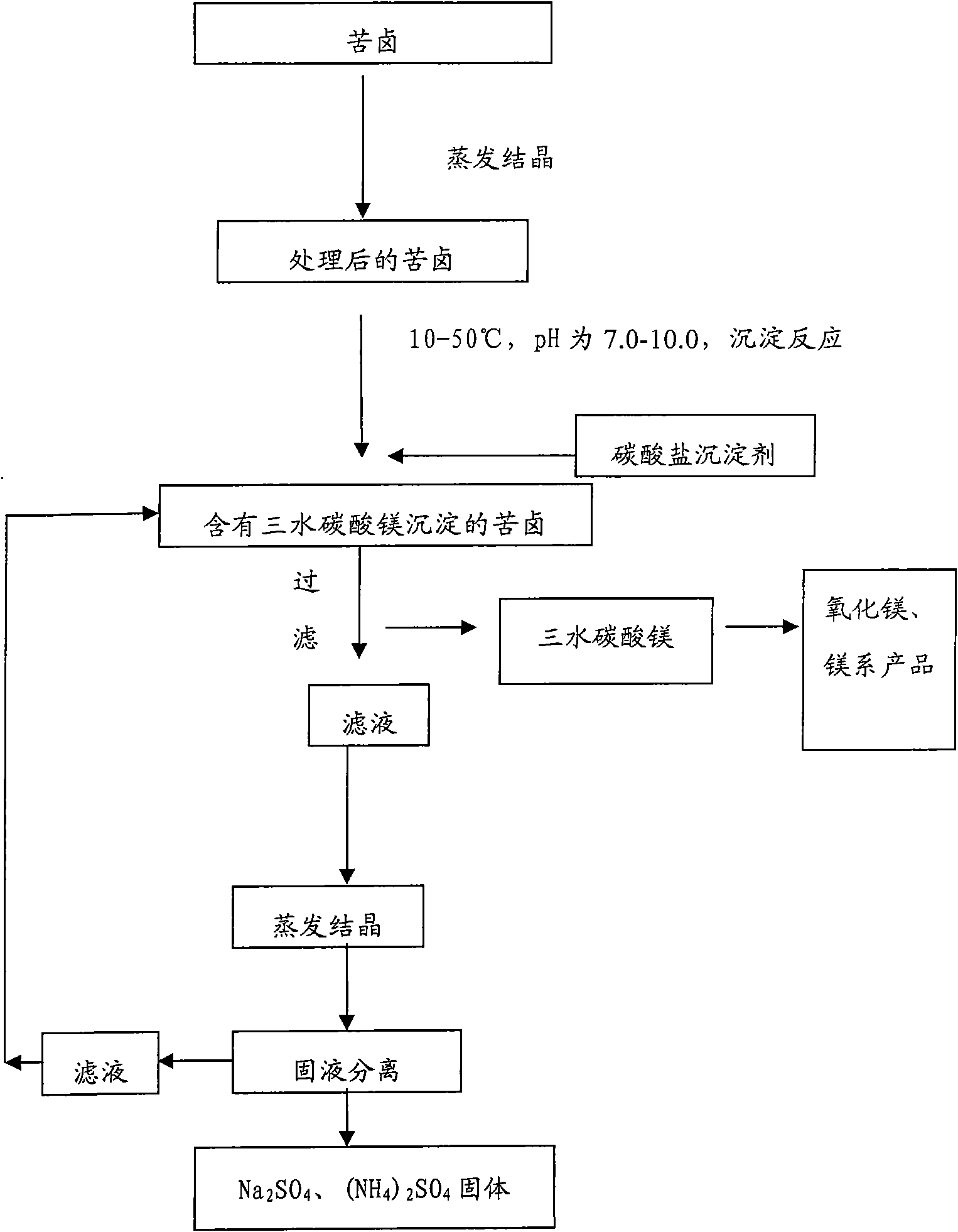
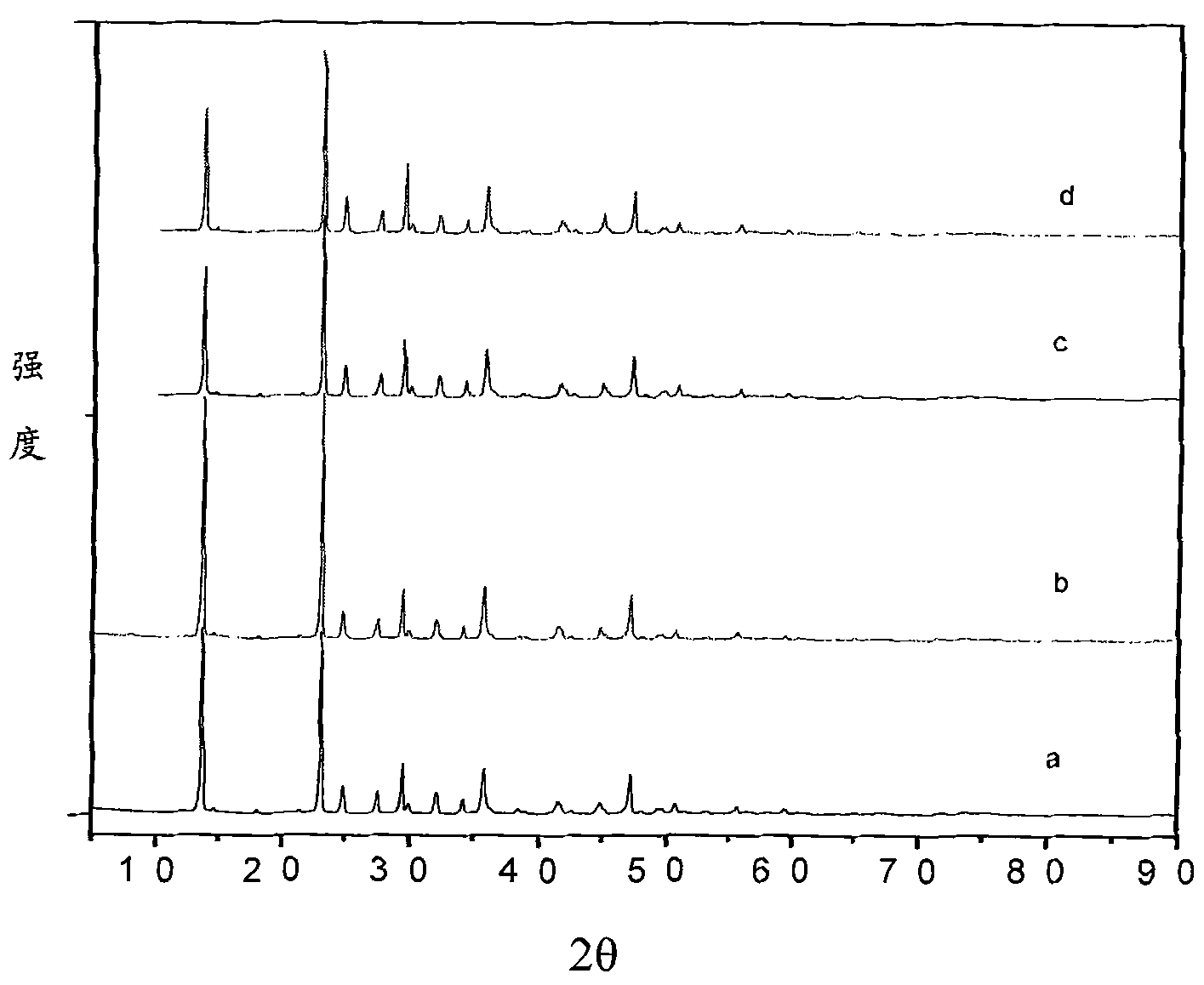
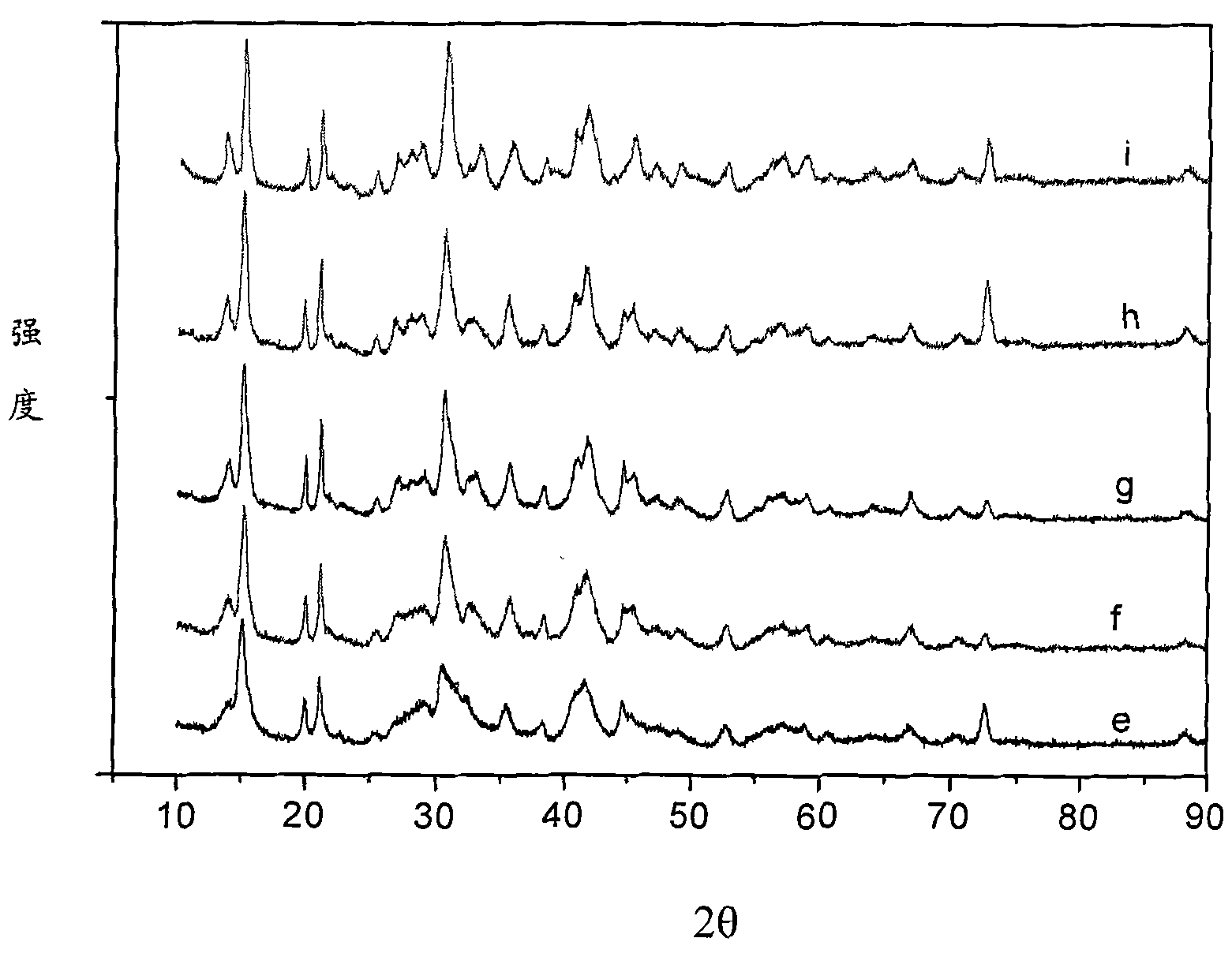
[0053] First, the bittern containing magnesium sulfate is evaporated and crystallized to remove the sodium salt and potassium salt, and deionized water is added to the bittern that removes the sodium salt and potassium salt to prepare 1000ml of magnesium sulfate with a concentration of 1mol / L. Bottom liquid, heated to keep the solution at 30°C, 1000ml of sodium carbonate solution with a concentration of 1.1mol / L was slowly added therein under stirring, and the adding speed was controlled to keep the pH value of the reaction solution at 8.5. After the reaction, continue to stir at room temperature for 2 hours, and age to generate needle-shaped magnesium carbonate trihydrate precipitates. The above precipitate is filtered, washed and dried to obtain a magnesium carbonate trihydrate product, which is calcined at 700° C. for 2 hours to obtain magnesium oxide whiskers. Mg in the resulting filtrate 2+ The content is 1.24g / L, the filtrate is evaporated, concentrated, crystallized, f...
Embodiment 2
[0055] First, the bittern containing magnesium sulfate is evaporated and crystallized to remove the sodium salt and potassium salt, and deionized water is added to the bittern that removes the sodium salt and potassium salt to prepare 1000ml of magnesium chloride with a concentration of 0.5mol / L. The bottom solution was heated to keep the solution at 30°C, and 48g of sodium carbonate solid powder was slowly added therein under stirring, and the addition rate was controlled to keep the pH value of the reaction solution at 9.0. After the reaction was completed, the stirring was continued at room temperature for 6 hours, and the needle-shaped magnesium carbonate trihydrate precipitate was formed by aging. The above precipitate was filtered and dried to obtain a magnesium carbonate trihydrate product, which was calcined at 700° C. for 3 hours to obtain magnesium oxide whiskers. Mg in the resulting filtrate 2+ The content is 1.10g / L, the filtrate is evaporated, concentrated, cryst...
Embodiment 3
[0057] First, the bittern containing magnesium sulfate is evaporated and crystallized to remove the sodium salt and potassium salt, and deionized water is added to the bittern that has removed the sodium salt and potassium salt, and the concentrations of magnesium sulfate and magnesium chloride are respectively 2mol / L. 1000ml of the reaction bottom solution was heated to keep the solution at 25°C, and 2100ml of sodium carbonate solution with a concentration of 1mol / L was slowly added therein under stirring, and the addition rate was controlled so that the pH value of the reaction solution was 8.3. After the reaction was completed, the stirring was continued at room temperature for 4 hours, and the needle-shaped magnesium carbonate trihydrate precipitate was formed by aging. The above precipitate was filtered and dried to obtain a magnesium carbonate trihydrate product, which was calcined at 700° C. for 4 hours to obtain magnesium oxide whiskers. Mg in the resulting filtrate 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com