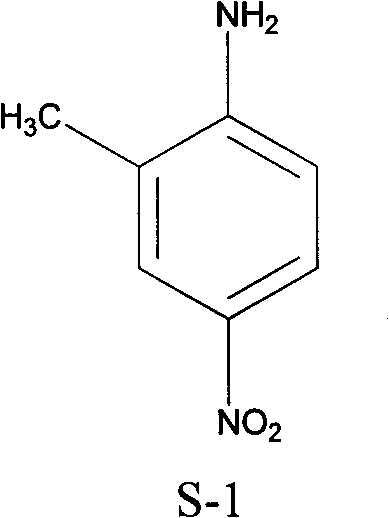
Preparation method of 2-methyl-4-nitrophenylamine
A technology for nitroaniline and o-toluidine, which is applied in the field of organic compound synthesis, can solve the problems of many side reactions, consumption of large alkali solution, and high requirements for reaction conditions, and achieves reduction of the generation of by-products and the discharge of waste acid. , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
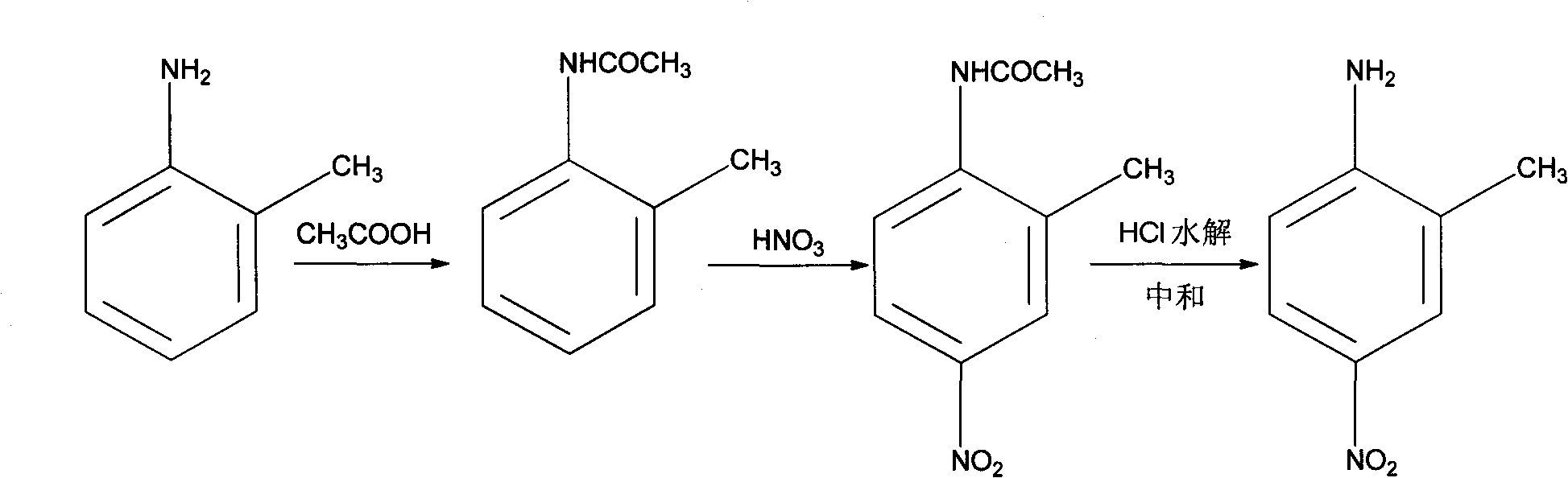
[0025] Example 1. A method for preparing 2-methyl-4-nitroaniline, using o-methylaniline as a starting material, the following steps are carried out in sequence:
[0026] (1) Add 45g (0.75mol) of acetic acid to a 250ml three-necked flask, add 32.1g (0.3mol) o-methylaniline dropwise, and control the feeding temperature not to exceed 60°C. After the dripping is completed, the temperature is raised to 100°C to react, and the generated water is continuously evaporated during the reaction, and the reaction is stopped after 4 hours.
[0027] (2) 32g (0.33mol) concentrated nitric acid (65%, Wt%, the same below) was slowly dropped into the reaction solution obtained in the above step (1), and the process was cooled by a water bath to keep the temperature below 30°C. Then, react at a constant temperature at 30°C for 5h; after the reaction is completed, pour the reaction solution into cold water at 10°C to precipitate a yellow solid, filter and dry to obtain a nitrated solid.
[0028] (3) Add ...
Embodiment 2
[0030] Example 2. A preparation method of 2-methyl-4-nitroaniline, using o-methylaniline as a starting material, the following steps were carried out in sequence:
[0031] (1) Add 45g (0.75mol) of acetic acid to a 250ml three-necked flask, add 32.1g (0.3mol) o-methylaniline dropwise, and control the feeding temperature not to exceed 60°C. After the dripping is completed, the temperature is raised to 100°C to react, and the generated water is continuously evaporated during the reaction, and the reaction is stopped after 4 hours.
[0032] (2) 32 g (0.33 mol) of concentrated nitric acid (65%) was slowly dropped into the above reaction liquid, and during the dropping process, it was cooled in an ice water bath, and the feeding temperature was controlled not to exceed 10°C. Then react at a constant temperature at 10°C for 5 hours. After the reaction is completed, the reaction solution is poured into cold water at 10°C to precipitate a yellow solid, which is filtered and dried to obtain ...
Embodiment 3
[0035] Example 3. A method for preparing 2-methyl-4-nitroaniline, using o-methylaniline as a starting material, the following steps were carried out in sequence:
[0036] (1) Add 45g (0.75mol) of acetic acid to a 250ml three-necked flask, add 32.1g (0.3mol) o-methylaniline dropwise, and control the feeding temperature not to exceed 60°C. After the dripping is completed, the temperature is raised to 100°C to react, and the generated water is continuously evaporated during the reaction, and the reaction is stopped after 4 hours.
[0037] (2) 32g (0.33mol) of concentrated nitric acid (65%) was slowly dropped into the above reaction liquid, and the water bath was cooled during the dropping process, and the feeding temperature was controlled not to exceed 40°C. Then react at a constant temperature at 40°C for 5 hours. After the reaction is completed, the reaction solution is poured into cold water at 10°C to precipitate a yellow solid, which is filtered and dried to obtain a nitrated so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com