Method for preparing organic-dissolvable photosensitive chitosan derivate
A technology of chitosan derivatives and organic dissolution is applied in the field of preparation of organic-soluble photosensitive chitosan derivatives, and can solve the problem of reducing the application value of chitosan derivatives, many by-products in the reaction, and high energy consumption, etc. problems, to achieve the effect of good UV absorption, saving raw materials and costs, and increasing the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
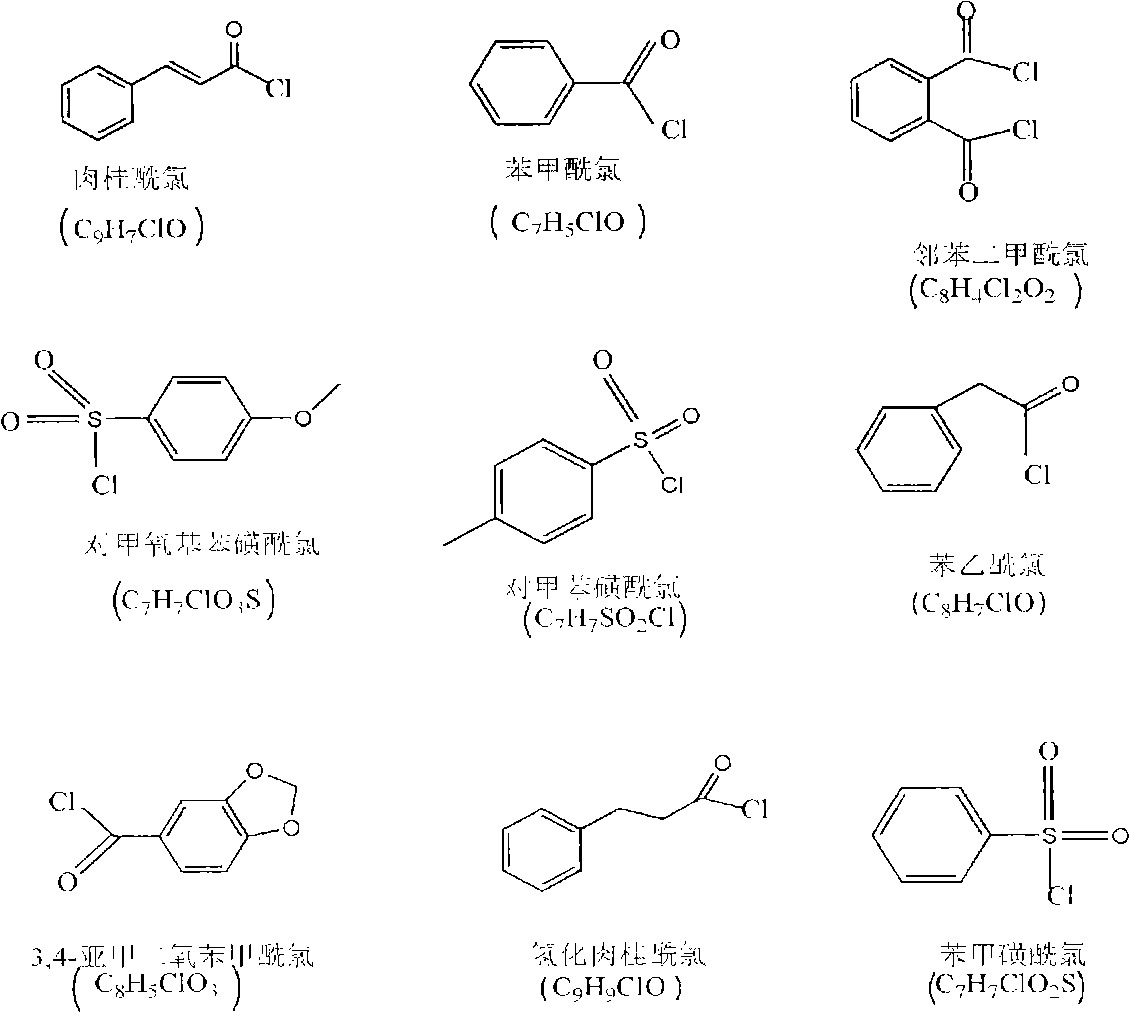
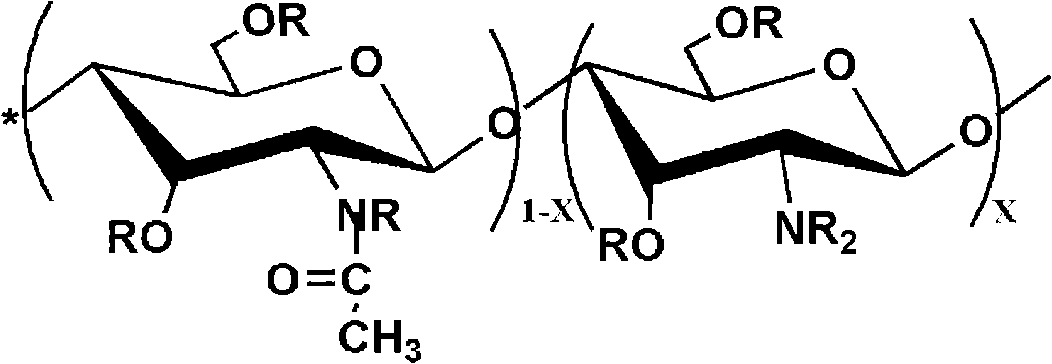
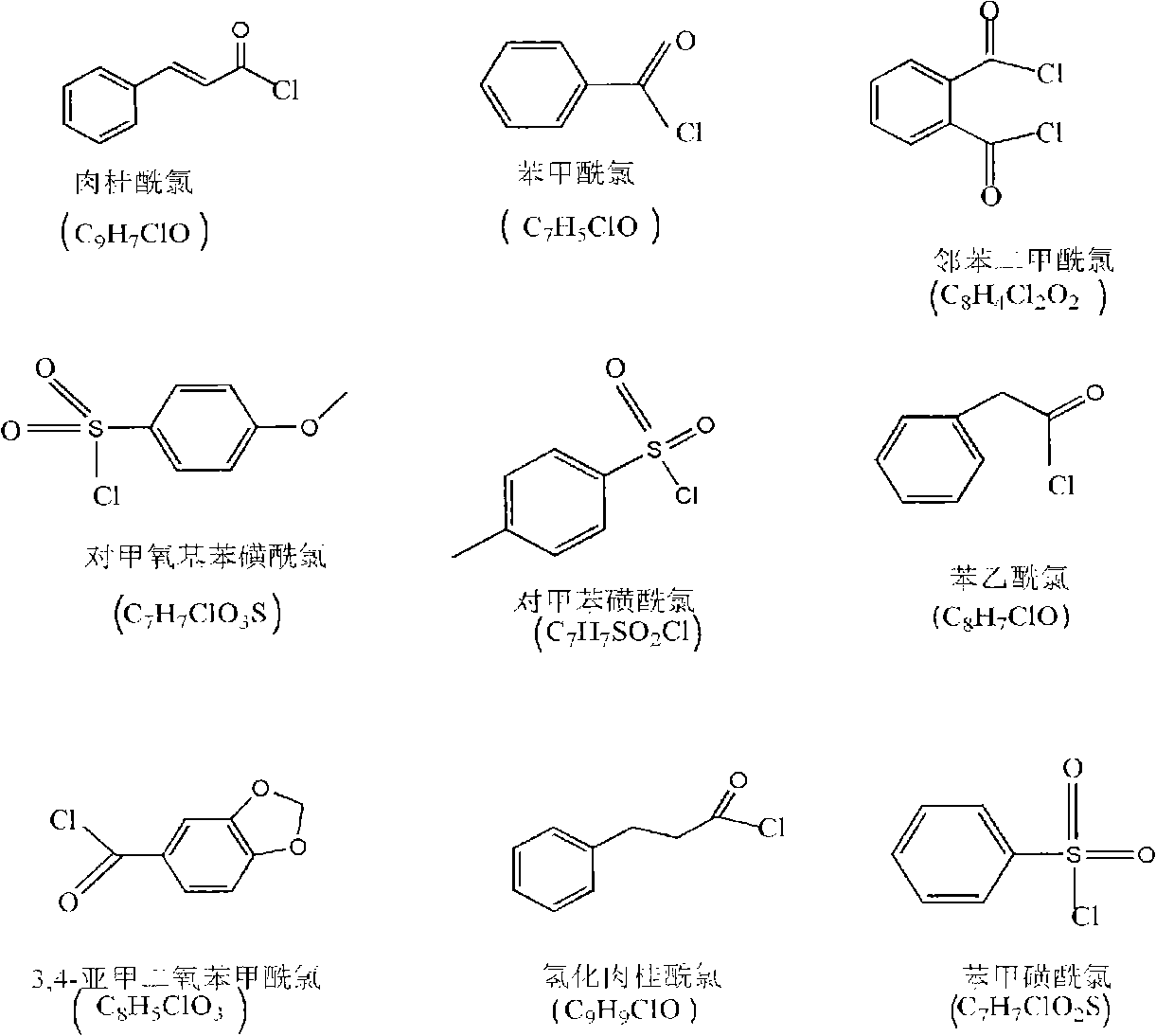
[0028] 2g chitosan (degree of deacetylation DP=80%, weight average molecular weight Mw=500,000) is added in the triethylamine of 70ml, stirs at room temperature, chitosan is evenly dispersed in triethylamine; Add cinnamoyl chloride In the carbon tetrachloride solution, be prepared with the carbon tetrachloride solution of cinnamoyl chloride, wherein the addition of photosensitive acyl chloride monomer is 0.5 times of the molar number of amino, imino and hydroxyl groups on chitosan; the photosensitive acyl chloride The carbon tetrachloride solution of quasi-monomer was slowly added dropwise in the chitosan solution, and kept stirring; after all the dropwise addition was completed, the reaction was continued for 6h, and after the reaction was completed, suction filtration was carried out to obtain a powdery solid, and then used Wash 3 times with 8wt% sodium bicarbonate solution, filter the washed product, and vacuum dry to obtain a photosensitive chitosan derivative with a substi...
Embodiment 2
[0034]2g chitosan (degree of deacetylation DP=70%, weight average molecular weight Mw=1,000,000) is added in the triethylamine of 70ml, stirs at room temperature, chitosan is evenly dispersed in triethylamine; Add it into the carbon tetrachloride solution to prepare the carbon tetrachloride solution of benzoyl chloride, wherein the addition amount of the photosensitive acyl chloride monomer is 2 times of the molar number of the amino group, imino group and hydroxyl group on the chitosan; Slowly add the carbon tetrachloride solution of the neutral acid chloride monomer into the chitosan solution dropwise, and keep stirring; after all the dropwise addition is completed, the reaction is continued for 4 hours, and after the reaction is completed, suction filtration is carried out to obtain a powdery solid. Then wash 3 times with 8wt% sodium bicarbonate, filter the washed product, and vacuum dry to obtain a photosensitive chitosan derivative with a degree of substitution of 1.6, whi...
Embodiment 3
[0040] 2g chitosan (degree of deacetylation DP=50%, weight average molecular weight Mw=100,000) is added in the triethylamine of 70ml, stirs at room temperature, chitosan is evenly dispersed in triethylamine; Add phenylsulfonyl chloride to carbon tetrachloride solution to prepare a carbon tetrachloride solution of p-methoxybenzenesulfonyl chloride, wherein the amount of photosensitive acyl chloride monomer is the amino group, imino group and hydroxyl group on chitosan 3 times the number of moles; slowly drop the carbon tetrachloride solution of the photosensitive acyl chloride monomer into the chitosan solution, and keep stirring; after all the drops are finished, continue to react for 6h, after the reaction is completed, carry out Suction filtration to obtain a powdery solid, then wash 3 times with 8wt% sodium bicarbonate, filter the washed product, and vacuum dry to obtain a photosensitive chitosan derivative with a degree of substitution of 2.3, which can be dissolved in ace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com