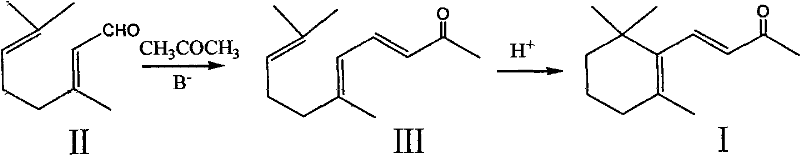
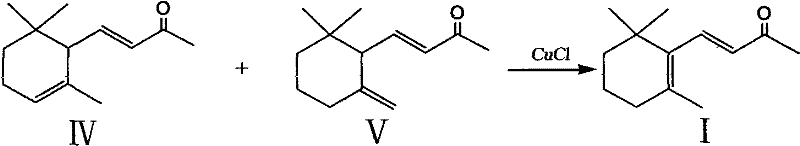
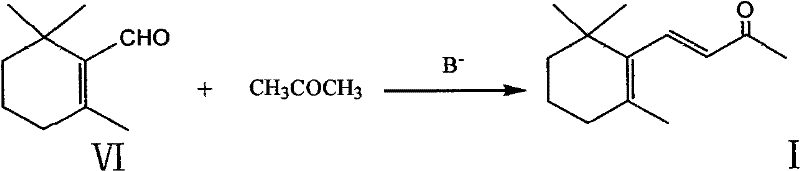Preparation method of beta-ionone
A technology of ionone and acetone, which is applied in the field of preparation of beta-ionone, can solve the problems of cumbersome process and difficult operation, and achieves the effects of simple operation, high yield and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of α-cyclic citral (VII)
[0030]
[0031] Dilute 45.6g (0.3mol) of citral with 45ml of dichloroethane and add it to a 250ml four-necked flask, keep warm in a water bath, and slowly drop into a solution of 30.0g of aniline in 30ml of dichloroethane while stirring at room temperature. After the dropwise addition was completed, the mixture was stirred for another half an hour, and the TLC reaction was completed (developing solvent: ethyl acetate:petroleum ether=1:3). The reaction mixture was dried with 8 g of anhydrous sodium sulfate, and the dried dichloroethane solution of citralimine could be directly used in the cyclization reaction. Add 120ml of 98% concentrated sulfuric acid and 140ml of dichloroethane into a 500ml four-necked bottle, mix and stir, cool in a cold bath to -20~-25°C, and slowly drop the imine solution prepared above into it under vigorous stirring. The temperature was controlled at about -20°C, the addition was comple...
Embodiment 2
[0036] Embodiment 2: Condensation reaction prepares β-ionone
[0037]In a 500ml four-necked flask, add 30.4g (0.2mol) of α-cyclocitral and 250g of acetone, then add 3ml of 5% aqueous sodium hydroxide solution, and stir the reaction at 35-45°C under nitrogen protection. After about 6 hours, the gas chromatography tracked that the raw material basically disappeared, and 1ml of acetic acid was added, and the acetone was recovered at normal pressure. After the acetone was recovered under normal pressure, the 85-89 ° C / 1mmHg fraction was collected by oil pump vacuum distillation to obtain 29.4 g of the product (93.5% in gas phase content), and the yield was 71.6%. The first portion 10.2 grams is a mixture of α-cyclic citral, β-cyclic citral, α-ionone, and β-ionone (gas phase content 15.5%: 29.5%: 26.3%: 29.1%), which can be applied mechanically to the next batch reaction. Product structure verification:
[0038] GC-MS (m / e): 192, 177 (100%), 162, 149, 135, 121, 107, 91, 77, 43; I...
Embodiment 3
[0041] Embodiment 3: the preparation of β-cyclic citral (VI)
[0042]
[0043] Dilute 0.3g KOH with 60ml of methanol and add it into a 250ml three-neck flask, and add 30.4g (0.2mol) of α-cyclocitral dropwise under stirring at room temperature. After the dropwise addition was completed, the stirring was continued for about 1 hour, and the reaction was followed by gas chromatography, and then 1.2 g of concentrated hydrochloric acid was added to terminate the reaction. The solvent was recovered, the residue was distilled under reduced pressure, and 25.5 g of 60-65°C / 1mmHg fraction was collected as a colorless transparent liquid with a gas phase content of 98.5% and a yield of 83.9%. Product structure verification:
[0044] GC-MS (m / e): 152, 137 (100%), 123, 109, 95, 91, 81, 67, 55, 43, 41; IR (v / cm -1 ): 1672 (-CHO, characteristic peak of aldehydes), 1612 (double bond);
[0045] 1 HNMR (δ, ppm, 400MHz, CDCl 3 ): 1.19 (s, 6H, -CH 3 ), 1.43-1.46 (m, 2H, CH 2 -C), 1.60-1.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com