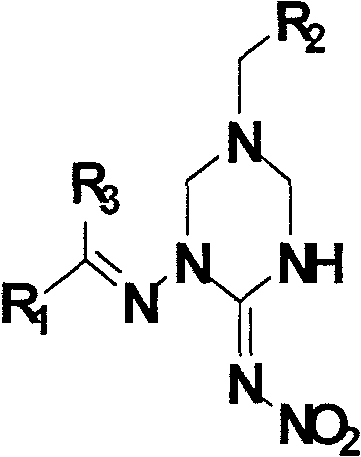
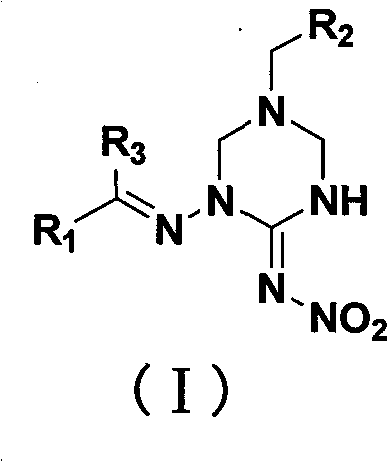
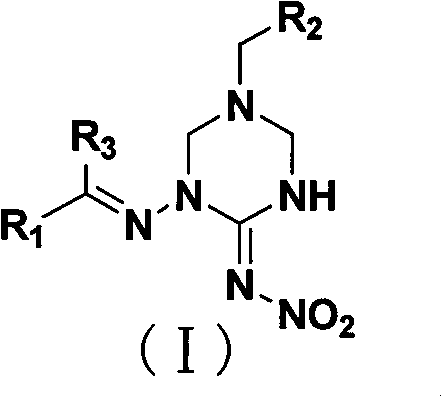
1,5-disubstituted hexahydrotriazine-2-N-nitroimine derivative
A technology of -2-N- and hexahydrotriazine, which is applied in the field of 1, can solve the problems of complex production process, low output and high cost, and achieve the effect of simple production process, high output and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of 1-(2-furylmethyleneamino)-5-(2-chloro-5-pyridylmethyl)-1,3,5-hexahydrotriazine-2-N-nitroimine (Ia):
[0059] (1) Put 5.0 g of nitroguanidine and 70 mL of water into a 250 mL three-necked bottle. The mixture was heated to 55°C under magnetic stirring, and 3.5 g of 85% hydrazine hydrate was added dropwise from the dropping funnel. Keep the temperature at 55-60°C and continue the reaction for 20min, to an orange-yellow clear liquid, rapidly cool with an ice-water bath, adjust the pH value to 5-6, continue to cool to 0°C, hold for 1h, and filter under reduced pressure to obtain a solid. Washed with a small amount of ice water to obtain 2.5 g of pale yellow powder (1-nitro-3-aminoguanidine) with a yield of 44%.
[0060] (2) 2.0 g of 1-nitro-3-aminoguanidine, 80 mL of anhydrous ethanol and 0.2 mL of glacial acetic acid were put into a 250 mL three-necked flask. The mixture was heated to 65° C. under magnetic stirring, and a mixed solution of 1.94 g of 2-furanca...
Embodiment 2
[0067] Preparation of 1-(2-furylmethyleneamino)-5-(2-tetrahydrofuranmethyl)-1,3,5-hexahydrotriazine-2-N-nitroimine Ib:
[0068] (1) Preparation of 1-nitro-3-aminoguanidine in the same manner as in step (1) of Ia.
[0069] (2) Preparation of 1-nitro-3-(2-furamethylene) aminoguanidine in the same manner as in step (2) of Ia.
[0070] (3) 2.0 g of 1-nitro-3-(2-furamethylene) aminoguanidine and 20 mL of acetonitrile were put into a 100 mL three-necked flask, and heated to 60° C. under magnetic stirring. A mixed solution of 1.03 g of 2-aminomethyltetrahydrofuran and 1.97 g of 37% aqueous formaldehyde solution was slowly added dropwise from the funnel. After the dropwise addition was completed, the temperature was maintained for 1 h. Column chromatography (petroleum ether:ethyl acetate=2:1) to obtain 1-(2-furamethyleneamino)-5-(2-tetrahydrofuranmethyl)-1,3,5-hexahydrotriazine-2 -N-Nitroimine 2.35. Yield 72%, melting point: 142°C-143°C.
[0071] Elemental analysis: Measured va...
Embodiment 3
[0076] Preparation of 1-(2-furylmethyleneamino)-5-(2-furylmethyl)-1,3,5-hexahydrotriazine-2-N-nitroimine Ic:
[0077] (1) Preparation of 1-nitro-3-aminoguanidine in the same manner as in step (1) of Ia.
[0078] (2) Preparation of 1-nitro-3-(2-furamethylene) aminoguanidine in the same manner as in step (2) of Ia.
[0079] (3) 2.0 g of 1-nitro-3-(2-furamethylene) aminoguanidine and 20 mL of acetonitrile were put into a 100 mL three-necked flask, and heated to 60° C. under magnetic stirring. A mixed solution of 0.99 g of 2-aminomethylfuran and 1.97 g of a 37% aqueous formaldehyde solution was slowly added dropwise from the funnel. After the dropwise addition was completed, the temperature was maintained for 1 h. Column chromatography (petroleum ether:ethyl acetate=2:1) to obtain 1-(2-furamethyleneamino)-5-(2-furanmethyl)-1,3,5-hexahydrotriazine-2 -N-nitroimine 2.23 g. Yield 69%, melting point: 114°C-117°C.
[0080] Elemental analysis: Measured value: C% 49.06 H% 4.41 N% 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com