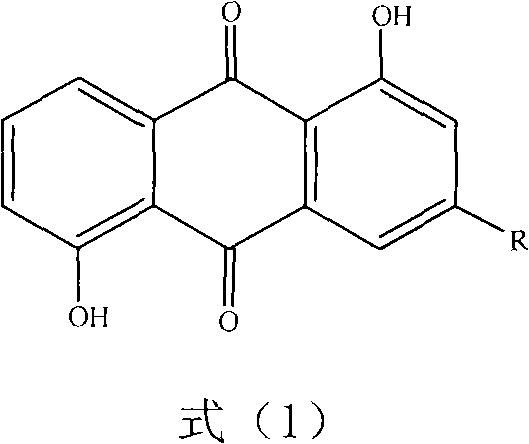
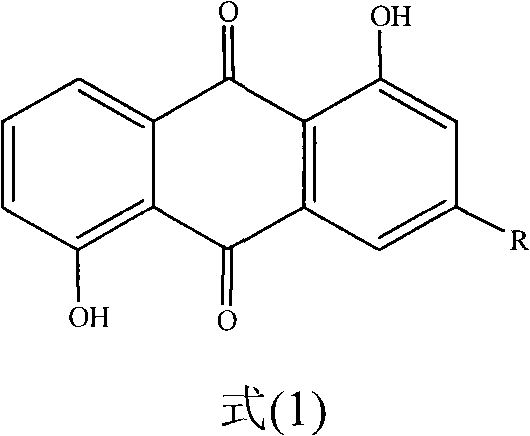
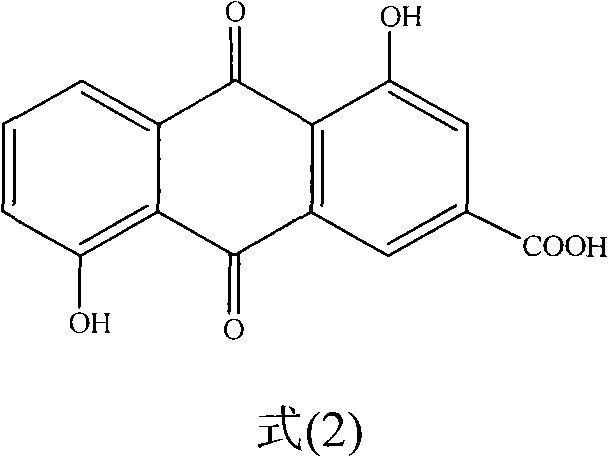
Anthraquinone compound and preparation method and medical application of lysine salt thereof
A compound, anthraquinone technology, applied in the field of research and development of new traditional Chinese medicines, can solve problems such as unsatisfactory effects, toxic side effects, etc., and achieve the effects of good crystal stability, good water solubility, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Preparation of catallic acid
[0110] Take 5 kg of dry coarse powder of the stem bark of the walnut catalpa, extract it continuously with 30% ethanol solution under reflux for 3 times, each time for 2 hours, combine the extracts, concentrate under reduced pressure to obtain a black extract, and then suspend it in deionized water A 2 L suspension was obtained, which was extracted three times with an equal volume of petroleum ether, chloroform, ethyl acetate, and n-butanol in sequence, and the extracted layers were combined and concentrated under reduced pressure. The chloroform extract (54 g) was subjected to vacuum silica gel column chromatography, and was eluted with a petroleum ether-ethyl acetate gradient to obtain 3 fractions of I-III, and yellow was precipitated in fraction II (Petroleum Ether:EtOAc / 70:30). Green amorphous powder, recrystallized in methanol to obtain catallic acid, and its structure identification data are shown in Table 12. ...
Embodiment 2
[0114] Preparation of Lysine Catalic Acid
[0115] Add 20mg catallic acid (taken from embodiment 1) and 32mg lysine (purchased from Beijing Qingshengda Chemical Technology Co., Ltd.) in reaction bottle, add 5ml double distilled water again, stirring reaction 20 hours, temperature is controlled at At 30°C, the final reaction solution turned into a clear and transparent liquid. Afterwards, put the reaction bottle into an ice bath, slowly add absolute ethanol dropwise to the reaction solution while stirring, about 6 drops / minute, and observe the change of the reaction solution at the same time, and stop adding anhydrous ethanol when purple-red crystals appear in the solution. Ethanol, 15.8ml of absolute ethanol was added at this time, and the addition time was 30 minutes, and then the reaction bottle was placed in a refrigerator at 4°C overnight, and a layer of purple-red crystals was observed on the wall of the bottle the next day, and the reaction solution...
experiment example 1
[0119] In Vitro Antitumor Experiment of Catalsic Acid
[0120] MTT method was used to carry out anti-tumor experiments in vitro. Each tumor cell was placed in a 96-well plate at a cell density of 5×10 4 pcs / ml; the next day, add catallic acid, the concentration is 100 μg / ml, 10 μg / ml, 1 μg / ml respectively, and the drug is diluted with DMSO, and a DMSO control group is set up at the same time, and each group is set up with 3 duplicate holes, and the volume of administration is 100 μl / ml. wells; cells in 5% CO 2 After continuing to incubate in a 37°C incubator for 44 hours, add 20 μl MTT (5 mg / ml) to each well and continue to incubate for 4 hours; discard the supernatant, add 100 μl DMSO to each well, vibrate for 600 s on a microplate reader, and detect the OD at 570 nm The independent experiment was repeated more than three times, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com