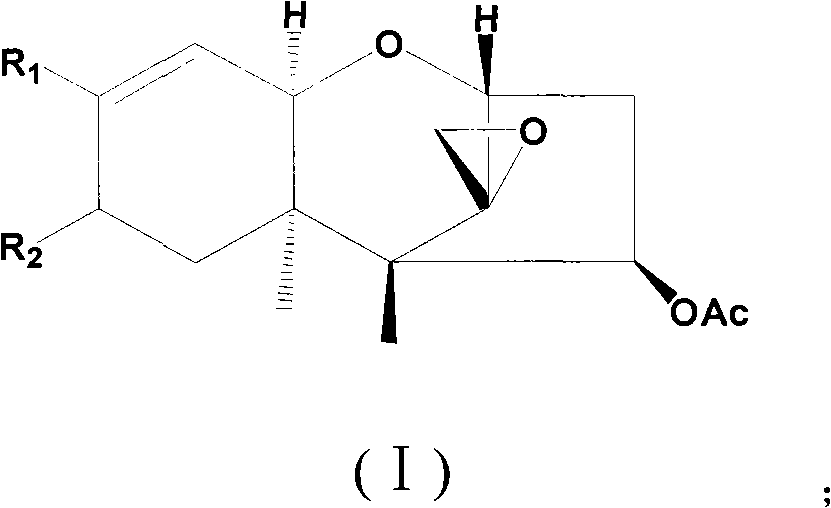
Trichodermin derivatives and application thereof in preventing and controlling pathogenic bacteria
A technology of trichoderma and phytopathogenic fungi, which is applied in the directions of chemicals, biocides, and applications for biological control, can solve the problems of chemical fungicides-resistant environment, food accumulation pollution, etc., and achieves excellent results, active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, a kind of synthetic method of trichoderma derivatives (compound 1), its reaction formula is:
[0038]
[0039] Concrete reaction process is as follows:
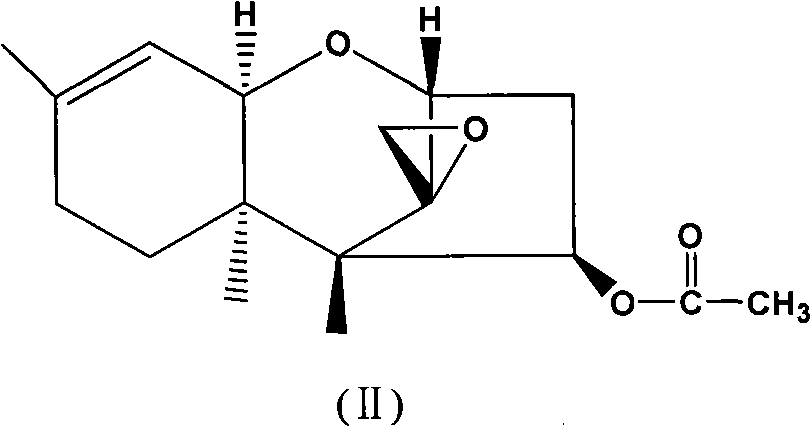
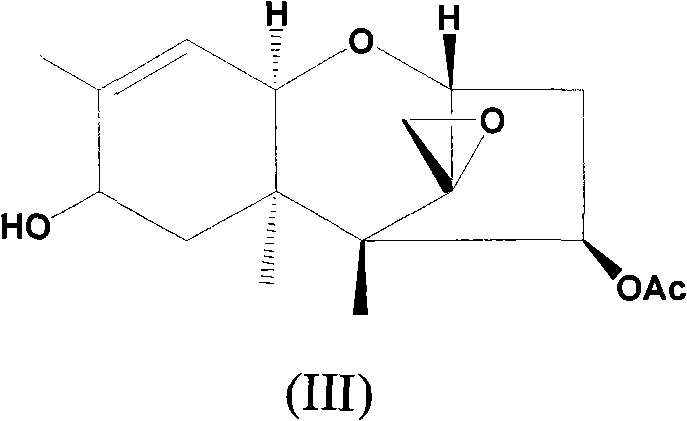
[0040] Add 4.0g (13.7mmol) trichodermain (structural formula II), 2.0g (18.0mmol) selenium dioxide and 80mL chloroform into the reaction flask, heat to reflux, and stir for 0.5h to react. The reaction solution was washed three times with water, anhydrous Na 2 SO 4 It was dried and concentrated to obtain 3.5 g of solid product, which was separated by silica gel column chromatography (eluent: ethyl acetate:petroleum ether=1:5, volume ratio) to obtain 0.8 g of compound 1 and 2.1 g of compound of formula III.
[0041] Structural data of compound 1: 1 H-NMR (500MHz, CDCl 3 , δppm): 9.537 (1H, s, -CHO), 6.612-6.598 (1H, m, -C=CH-), 5.611-5.588 (1H, d, -COOCH-), 3.891-3.880 (2H, m, -O-CH-CH 2 -&-C=C-CH-O-), 3.126-3.118 (1H, d, -O-CH 2 ), 2.857-2.849 (1H, d, -O-CH 2 ), 2.600-2.554 (1H, m, -COOCH-CH 2 ...
Embodiment 2
[0044] Embodiment 2, a kind of synthetic method of trichomycin derivative (compound 2), its reaction formula is:
[0045]
[0046] At room temperature (10~30 ℃), add 1.0g (3.2mmol) compound of formula III, 0.6g (6.0mmol) triethylamine and 80mL dichloromethane that embodiment 1 method gained in reaction bottle, dropwise add 664mg (4.0mL) mmol) phenylacryloyl chloride, stirred and reacted for 0.5h. The reaction solution was washed three times with 1% hydrochloric acid, and then washed with saturated NaHCO 3 The solution was washed twice, and finally washed twice with water, anhydrous Na 2 SO 4 It was dried and concentrated to obtain 1.38 g of solid product, which was separated and purified by silica gel column (eluent: ethyl acetate:petroleum ether=1:10) to obtain 1.09 g of compound 2.
[0047] Structural data of compound 2: 1 H-NMR (500MHz, CDCl 3 , δppm): 7.729-7.697 (1H, d, Ph-CH=CH-), 7.545-7.394 (5H, m, Ph-H 5 ), 6.458-6.426 (1H, d, Ph-CH=CH-), 5.615-5.604 (1H, m, ...
Embodiment 3
[0048] Embodiment 3, a kind of synthetic method of trichomycin derivative (compound 4), its reaction formula is:
[0049]
[0050] Add 1.0g (3.2mmol) of the compound of formula III, 0.6g (6.0mmol) of triethylamine and 80mL of methylene chloride obtained in the method of Example 1 into the reaction flask at room temperature (10-30°C), and then add dropwise 0.40g ( 4.3mmol) propionyl chloride, stirred and reacted for 0.5h. The reaction solution was washed three times with 1% hydrochloric acid, and then washed with saturated NaHCO 3 The solution was washed twice, and finally washed twice with water, anhydrous Na 2 SO 4 It was dried and concentrated to obtain 1.22 g of solid product, which was separated and purified through a silica gel column (eluent: ethyl acetate:petroleum ether=1:10) to obtain 0.96 g of compound 4.
[0051] Structural data of compound 4:
[0052] 1 H-NMR (500MHz, CDCl 3 , δppm): 5.571-5.554 (1H, m, -COOCH-), 5.538-5.515 (1H, m, -C=CH-), 5.378-5.345 (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com