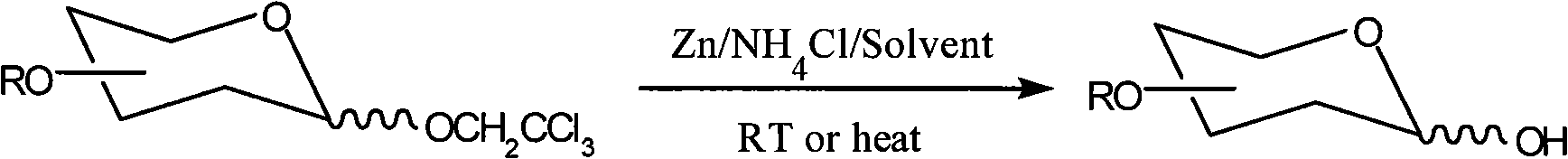Method for removing trichloroethyl of glucoside
A technology of trichloroethyl and trichloroethyl glycosides, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of limited method expansion, narrow application scope, long reaction time, etc. The effect of wide range, short reaction time and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Take 2,2,2-trichloroethyl-β-O-tetraacetylglucoside 80mg (0.167mmol), Zn powder 87mg (1.338mmol) and NH 4 Cl 27mg (0.504mmol), sequentially added to 2ml of analytically pure ethanol, and then heated to reflux for trichloroethyl removal reaction, the heating temperature is 80°C, the reaction time is 5min, the reaction product is filtered and concentrated under reduced pressure Finally, 57.2 mg of 2,3,4,6-O-tetraacetylglucose was obtained, and the yield was 98.5%.
[0020] The product 2,3,4,6-O-tetraacetylglucose obtained from the above-mentioned examples is carried out 1 H-NMR analysis, the test data are as follows:
[0021] 1 H NMR (500MHz, CDCl 3 ): δ5.55(t, J=10Hz, 0.75H), 5.47(d, J=4Hz, 0.73H), 5.26(t, J=10Hz, 0.3H), 5.09(t, J=10Hz, 1H) , 4.86-4.92(m, 1H), 4.75(d, J=8Hz, 0.27H), 4.22-4.30(m, 2H), 4.12-4.18(m, 1.4H), 3.75(m, 0.3H), 2.00 -2.20 (4s, 12H).
Embodiment 2
[0023] Take 2,2,2-trichloroethyl-β-O-tetraacetylmannoside 80mg (0.167mmol), Zn powder 65mg (1.002mmol) and NH 4 Cl 27mg (0.504mmol), sequentially added to 5ml of analytically pure acetone, then heated to reflux for trichloroethyl removal reaction, the heating temperature was 60°C, the reaction time was 5min, the reaction product was filtered and concentrated under reduced pressure Finally, 56.9 mg of 2,3,4,6-O-tetraacetylmannose was obtained, and the yield was 90%.
[0024] The product 2,3,4,6-O-tetraacetylmannose obtained from the above-mentioned examples was carried out 1 H-NMR analysis, the test data are as follows:
[0025] 1 H NMR (500MHz, CDCl 3 ): δ5.47(dd, J=10, 3Hz, 1H), 5.29(m, 3H), 4.25(m, 2H), 4.14(d, J=10H, 1H), 2.0-2.18(4s, 12H) .
Embodiment 3
[0027] Take 2,2,2-trichloroethyl-β-O-tetrabenzylgalactoside 60mg (0.089mmol), Zn powder 46mg (0.714mmol) and NH 4 Cl 38.1mg (0.712mmol) was added to 2ml of analytically pure ethanol in sequence, and then heated to reflux for trichloroethyl removal reaction. The heating temperature was 50°C and the reaction time was 1.5h. The reaction product was filtered and reduced After concentrated under reduced pressure, 49.2 mg of 2,3,4,6-O-tetrabenzylgalactose was obtained, and the yield was 95.0%.
[0028] The product 2,3,4,6-O-tetrabenzylgalactose obtained from the above-mentioned examples is carried out 1 H-NMR analysis, the test data are as follows:
[0029] 1 HNMR (500MHz, CDCl 3 ): δ7.35(m, 30H), 5.28(t, 0.93H), 5.0~4.4(m, 12.3H), 415(t, 0.88H), 4.04(dd, 0.91H), 3.89(m, 1.45 H), 3.76(m, 0.55H), 3.6~3.5(m, 4H), 3.11(d, 0.5H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com