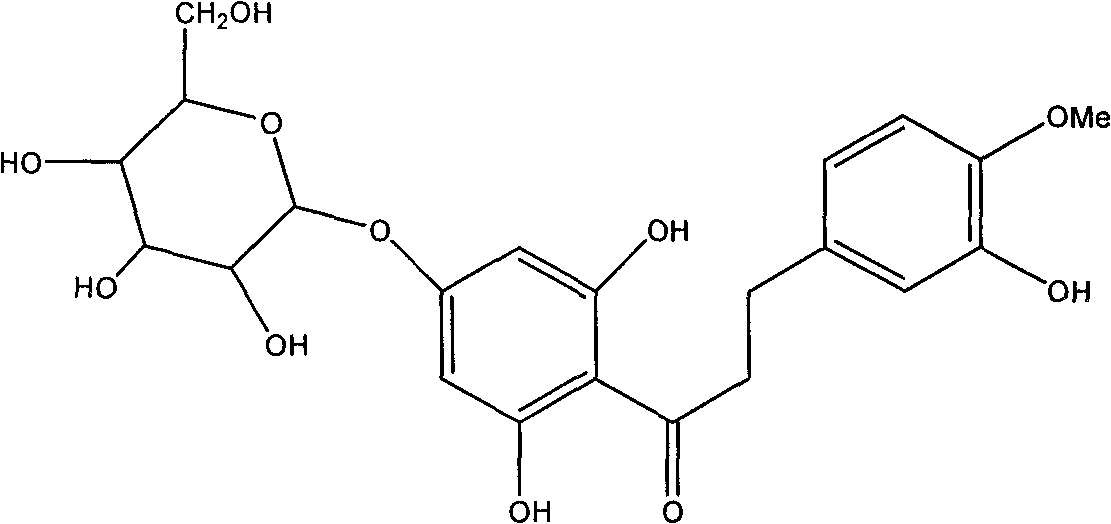
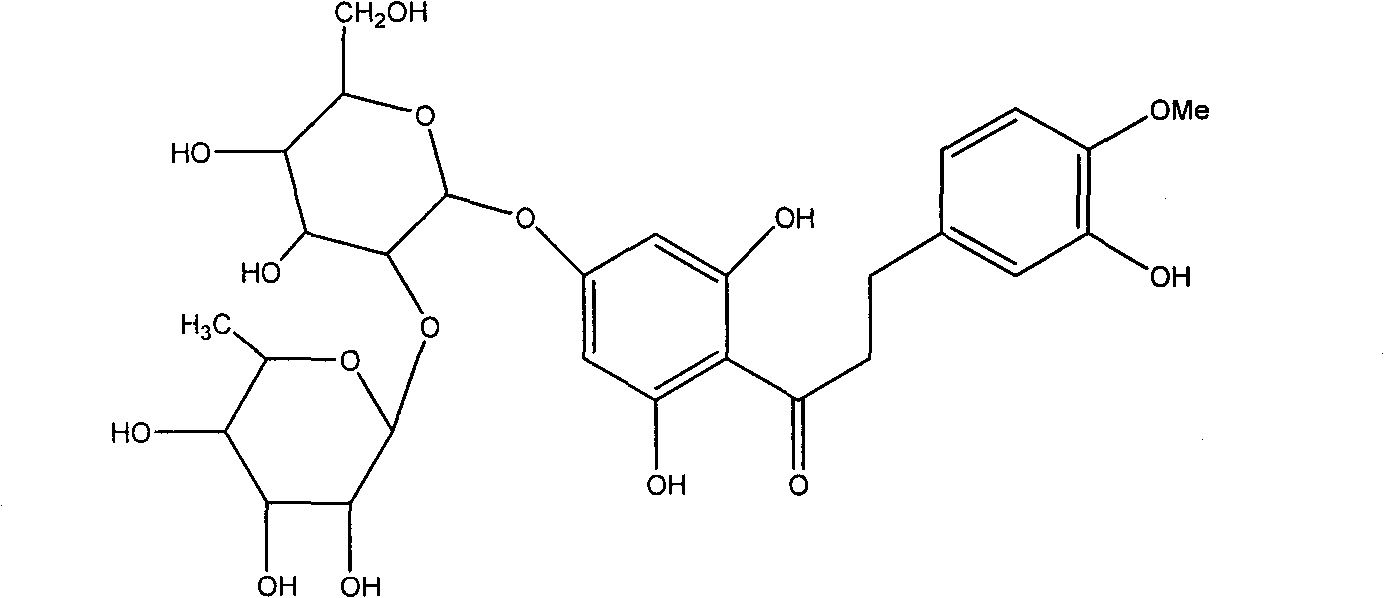
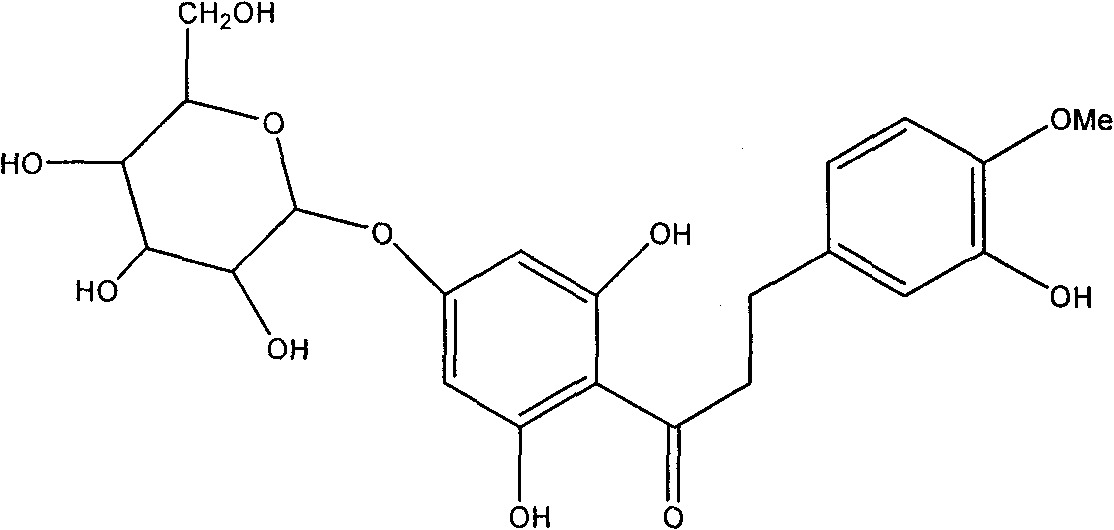
Hesperetin dihydrochalcone-7-O-glucoside and preparation method and application thereof
A technology for hesperetin dihydrochalcone and glucoside, which is applied in the field of hesperetin dihydrochalcone-7-O-glucoside and its preparation, can solve problems such as limited effect, and achieves the purpose of expanding the scope of use. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 2.0 g of citric acid was dissolved in 80 ml of distilled water, and its pH value was adjusted to 6.0 with 30% NaOH solution. Add 1g of neohesperidin dihydrochalcone and 0.01g of naringinase (enzyme activity 860u / g) to the above mixed solution, stir and react at 40°C for 24h, TLC tracking shows that neohesperidin dihydrochalcone The ketone moiety is converted to hesperetin dihydrochalcone-7-O-glucoside. After the reaction, heat the reaction mixture to 80°C to inactivate the enzyme, concentrate the solution, pass through the AB-8 macroporous resin column, wash the column with distilled water to remove sugar and inorganic salts, and then wash the column with 40% ethanol solution , concentrated and spin-dried to obtain 0.9 g of the product, namely hesperetin dihydrochalcone-7-O-glucoside.
Embodiment 2
[0033] Dissolve 2.0 g of citric acid in 80 ml of distilled water, adjust its pH value to 6.6 with 30% NaOH solution, then add 24 ml of ethyl acetate solution and stir to mix. Add 1g neohesperidin dihydrochalcone and 0.01g naringinase (enzyme activity 860u / g) to the above mixed solution, stir and react at 40°C for 30h, TLC tracking shows that neohesperidin dihydrochalcone Ketones are mostly converted to hesperetin dihydrochalcone-7-O-glucoside. After the reaction is over, heat the reaction mixture to 80°C to inactivate the enzyme, concentrate the solution, pass through the AB-8 macroporous resin column, wash the column with distilled water to remove sugar and inorganic salts, and then wash the column with 30% ethanol solution , concentrated and spin-dried to obtain 1.3 g of the product, namely hesperetin dihydrochalcone-7-O-glucoside.
Embodiment 3
[0035] Dissolve 2.0 g of citric acid in 80 ml of distilled water, adjust its pH value to 7.0 with 15% KOH solution, then add 20 ml of ethyl acetate solution and stir to mix. Add 1g of neohesperidin dihydrochalcone and 0.02g of naringinase (enzyme activity 860u / g) to the above mixed solution, stir and react at 35°C for 24h, TLC tracking shows that neohesperidin dihydrochalcone Ketones are mostly converted to hesperetin dihydrochalcone-7-O-glucoside. After the reaction, heat the reaction mixture to 80°C to inactivate the enzyme, concentrate the solution, pass through the AB-8 macroporous resin column, wash the column with distilled water to remove sugar and inorganic salts, and then wash the column with 40% ethanol solution , concentrated and spin-dried to obtain 1.7 g of the product, which was decolorized by activated carbon and crystallized from a mixed solvent of ethanol / water to obtain 1.1 g of white crystal hesperetin dihydrochalcone-7-O-glucoside, the content of which was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com