Metal ion doping and carbon coating jointly modified lithium ion battery anode material
A lithium-ion battery, metal ion technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as hindering lithium ion deintercalation, unfavorable charging and discharging process, difficulty in lithium ion diffusion, etc., to improve electronic conductivity and ionic Effect of diffusion coefficient, improving specific capacity of high current discharge and cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
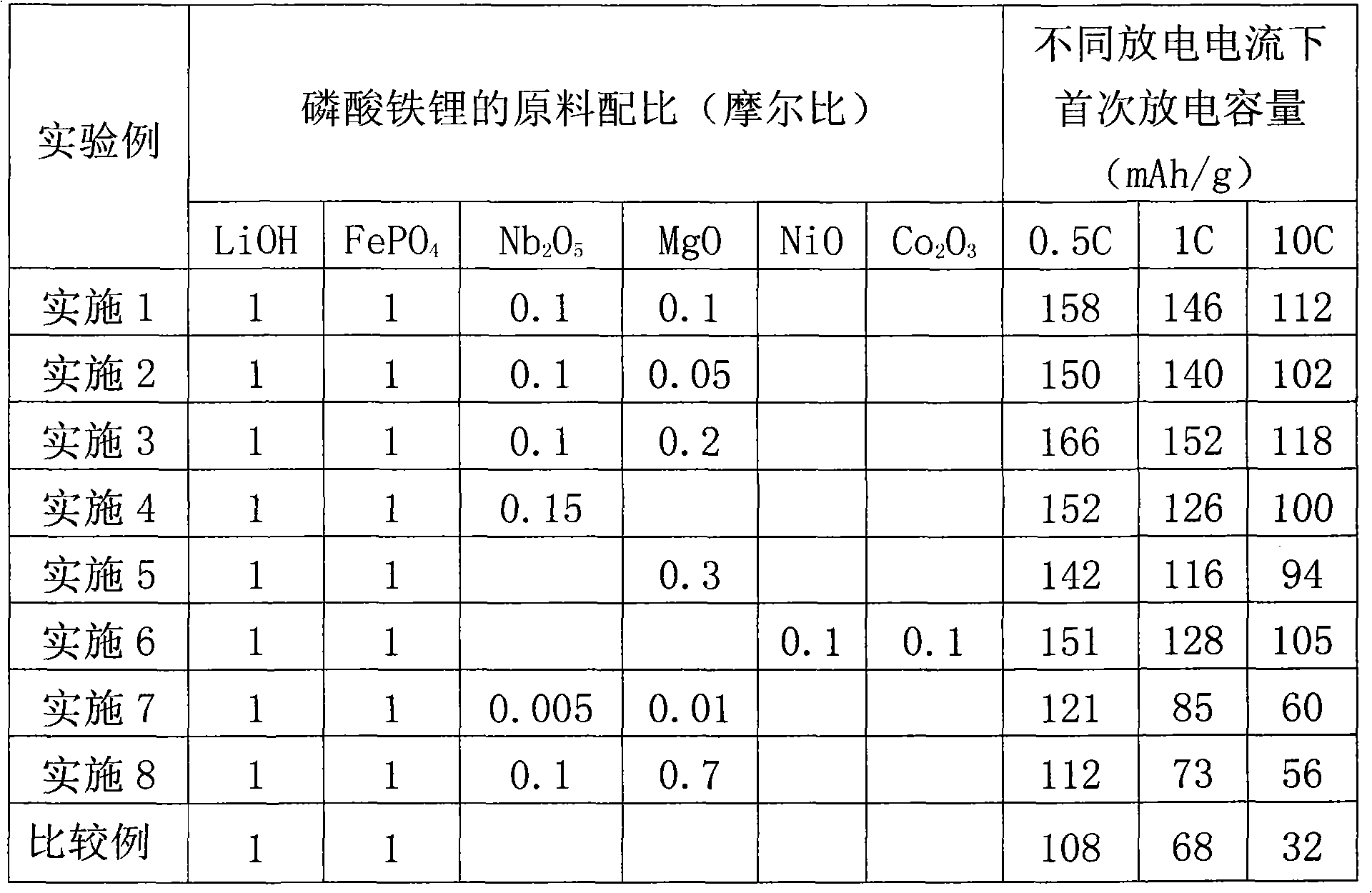
[0017] Analytical reagent LiOH·H 2 O, FePO 4 4H 2 O, Nb 2 o 5 and MgO as raw materials, and then accurately weigh LiOH, FePO according to the molar ratio of 1:1:0.1:0.1 4 , Nb 2 o 5 and MgO, then add expanded graphite with a molar ratio of 0.05 as a reducing agent and carbon source, add an appropriate amount of absolute ethanol as a grinding aid, mix well and place it in a ball mill jar for high-speed ball milling, with a ball-to-material ratio of 6:1. The ball-milled samples were dried at 60°C for 6 hours, and then 2 (The flow rate is 1L / min), heated to 600°C at a heating rate of 10°C / min under protection, kept for 4 hours, and then naturally cooled to room temperature to obtain carbon-coated Li 0.7 Mg 0.1 Nb 0.2 FePO 4 .
[0018] The prepared sample, activated carbon and polytetrafluoroethylene (PTFE) were mixed evenly according to the mass ratio of 80:15:5, and an appropriate amount of absolute ethanol was added dropwise as a dispersant, and ground for 1 hour. T...
Embodiment 2
[0020] Analytical reagent LiOH·H 2 O, FePO 4 4H 2 O, Nb 2 o 5 and MgO as raw materials, and then accurately weigh LiOH, FePO according to the molar ratio of 1:1:0.1:0.05 4 , Nb 2 o 5 and MgO, then add expanded graphite with a molar ratio of 0.05 as a reducing agent and carbon source, add an appropriate amount of absolute ethanol as a grinding aid, mix well and place it in a ball mill jar for high-speed ball milling, with a ball-to-material ratio of 6:1. The ball-milled samples were dried at 60°C for 6 hours, and then 2 (The flow rate is 1L / min), heated to 600°C at a heating rate of 10°C / min under protection, kept for 4 hours, and then naturally cooled to room temperature to obtain carbon-coated Li 0.7 Mg 0.1 Nb 0.2 FePO 4 . The preparation method of electrode is identical with embodiment 1, Li 0.7 Mg 0.1 Nb 0.2FePO 4 The charge and discharge capacities of the electrodes under the conditions of 0.5C, 1C and 10C are shown in Table 1, respectively.
Embodiment 3
[0022] Analytical reagent LiOH·H 2 O, FePO 4 4H 2 O, Nb 2 o 5 and MgO as raw materials, and then accurately weigh LiOH, FePO according to the molar ratio of 1:1:0.1:0.2 4 , Nb 2 o 5 and MgO, then add expanded graphite with a molar ratio of 0.05 as a reducing agent and carbon source, add an appropriate amount of absolute ethanol as a grinding aid, mix well and place it in a ball mill jar for high-speed ball milling, with a ball-to-material ratio of 6:1. The ball-milled samples were dried at 60°C for 6 hours, and then 2 (The flow rate is 1L / min), heated to 600°C at a heating rate of 10°C / min under protection, kept for 4 hours, and then naturally cooled to room temperature to obtain carbon-coated Li 0.6 Mg 0.2 Nb 0.2 FePO 4 . The preparation method of electrode is identical with embodiment 1, Li 0.6 Mg 0.2 Nb 0.2 FePO 4 The charge and discharge capacities of the electrodes under the conditions of 0.5C, 1C and 10C are shown in Table 1, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com