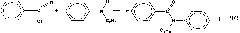Formula of working liquid for hydrogen peroxide production based on anthraquinone process
A hydrogen peroxide, working fluid technology, applied in peroxide/peroxyhydrate/peroxyacid/superoxide/ozonide, inorganic chemistry, chemical instruments and methods, etc., can solve the problem of high density and achieve The effect of reducing the degradation rate of anthraquinone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] This example describes the synthesis of N-phenyl N-ethylbenzamide (BEA).
[0018] The synthesis of BEA is carried out by N-ethylaniline and benzoyl chloride in the presence of excess NaOH according to the following reaction formula:
[0019]
[0020] Inject 69.3 grams of colorless N-ethylaniline (0.57 moles, molecular weight 121.6) and 176 grams (0.9 moles, about excess 50%) of 20% NaOH solution in a 500 mL three-necked flask with a thermometer and a dropping funnel. Bottle is immersed in the water bath that is placed on the electrothermal electromagnetic stirrer, benzoyl chloride 69.6mL (84.3 grams, 0.6 mole, specific gravity 1.23, about excess 5%, molecular weight 140.5) inject in the dropping funnel, stirring, and control reaction temperature At about 60°C, slowly add benzoyl chloride dropwise (reaction exothermic), and the drop is completed in about 40 minutes. Continue to stir at this reaction temperature for 30 minutes to complete the reaction and hydrolyze the...
Embodiment 2
[0023] The hydrogenation experiments were carried out in a magnetically stirred fixed-bed glass reactor. Charge 40ml Pd-Al in the reactor 2 o 3 Catalyst, use H at normal pressure, 65-80°C 2 :N 2 = After 24 hours of activation of the catalyst with a 3:1 hydrogen-nitrogen mixture, inject 240ml of working fluid into the reactor, keep the constant temperature at 50°C and continuously feed the above-mentioned atmospheric pressure hydrogen-nitrogen mixture with a gas volume of 1L / min for hydrogenation, and the magnetic stirring speed It is sufficient to ensure that the flow rate of the working fluid through the catalyst layer is about 200ml / min. Take out a small amount of liquid from the reaction system regularly to oxidize with oxygen, extract the oxidized liquid with pure water, and use potassium permanganate titration to measure H 2 o 2 content. H when precipitation occurs in the working solution 2 o 2 The content is used as the limit hydrogen effect of the working fluid....
Embodiment 3
[0026] Same as Example 2, just change the composition of the working solution into: solvent: heavy aromatics 75% (v / v), trioctyl phosphate 17% (v / v), BEA8% (v / v); working carrier: working solution Total quinones 182g / L, of which BAQ154g / L, H 4 BAQ 28g / L. Hydrogenation result (precipitation) is hydrogen effect: 12.13gH 2 o 2 / L, the density of the working fluid is 0.9320g / L measured at 30°C by the density bottle method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com