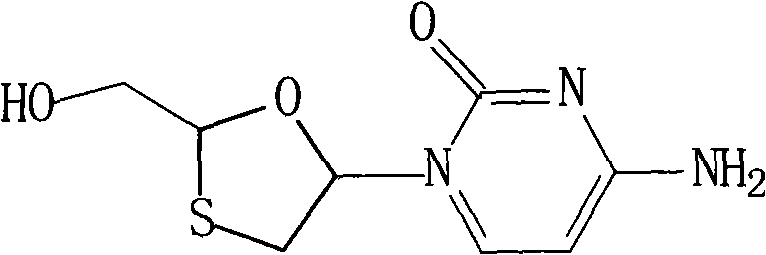
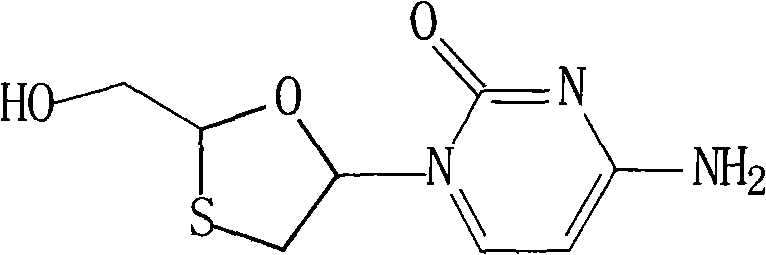
Lamivudine compound and novel preparation method thereof
A compound and intermediate technology, applied in the field of lamivudine compound and its new production method, can solve the problems of low yield and long synthesis steps, and achieve the effect of high yield, high purity and easy purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthesis of embodiment 1 lamivudine
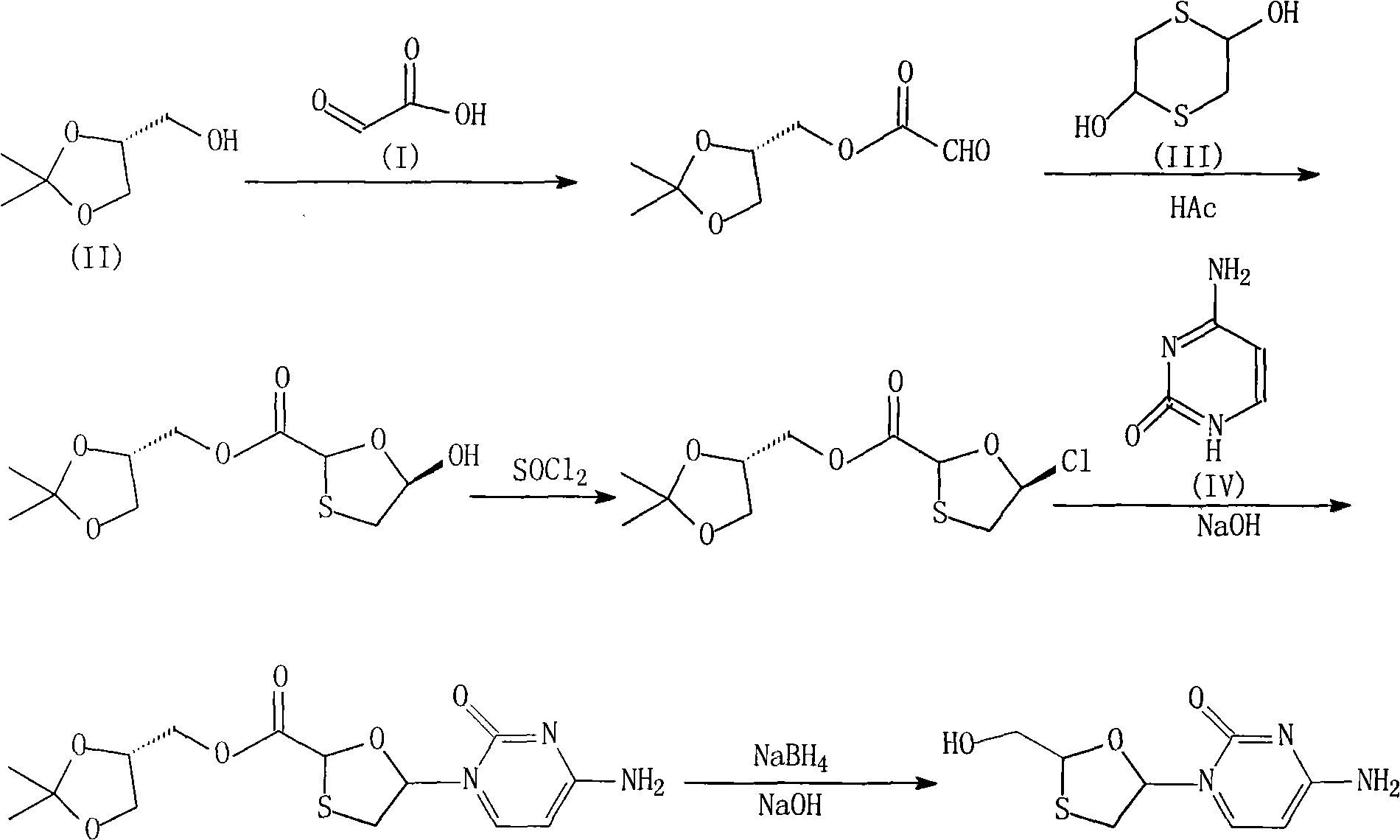
[0046] (1) Synthesis of glyoxylic acid-R-(-)-glycerol acetonide
[0047] Mix 74g of glyoxylic acid, 300ml of isopropyl ether, 132g of R-(-)-glycerol acetone and 15g of p-toluenesulfonic acid, heat and stir at room temperature until the solids are completely dissolved, then reflux for 10 hours, then cool to room temperature, filter, and separate Washed three times with 100ml of water, collected the organic phase, dried with anhydrous sodium sulfate for 12 hours, distilled the solvent under reduced pressure until the solid was precipitated, then added 400ml of petroleum ether, frozen, and filtered to obtain 173g of white solid, with a yield of 92%;
[0048] (2) Synthesis of (1R, 5R)-5-hydroxy-[1,3]-oxathiolane-2-carboxylic acid-R-(-)-glycerol acetonide
[0049] Mix 94g of glyoxylic acid-R-(-)-glycerol acetonide, 1000ml of toluene, 10ml of acetic acid, and 76g of 2,5-dihydroxy-1,4-dithiane, stir and reflux for 3 hours, and filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com