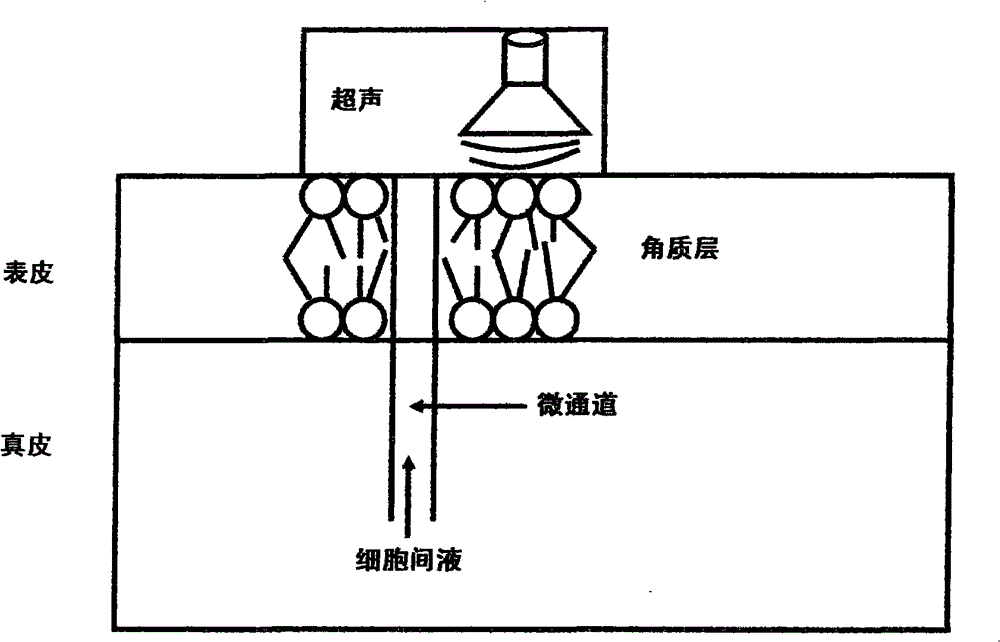
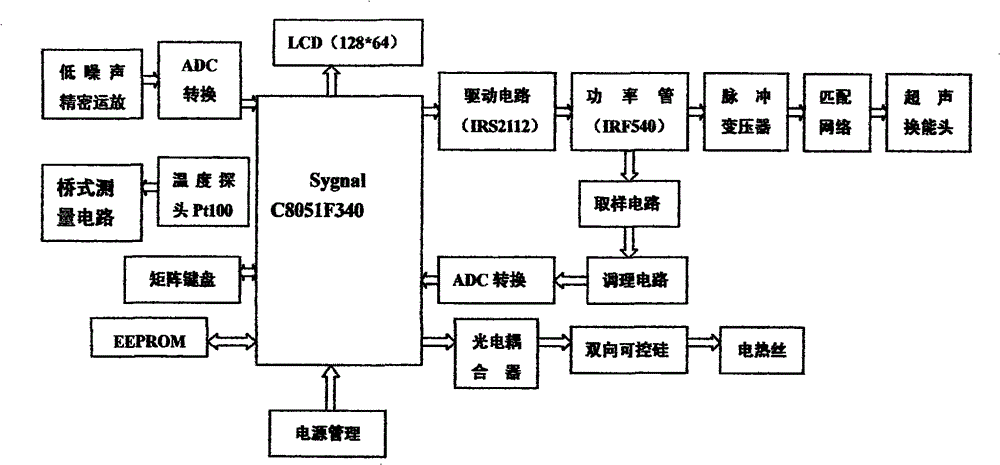
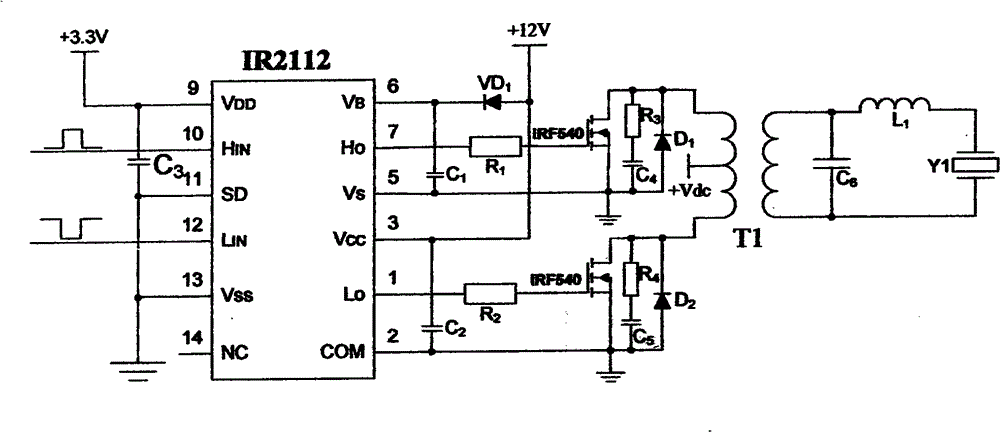
Rapid transdermal delivery system of local anesthetics
A transdermal drug delivery system and a technology for local anesthetics, which can be used in drug devices, other medical devices, etc., to solve problems such as unsatisfactory anesthesia effect and long onset time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of biodegradable nanoparticle gel with lidocaine as an example
[0024] The preparation steps of the nano-controlled release preparation loaded with local anesthetic lidocaine:
[0025] a. take stannous octoate as a catalyst, adopt PEG 4000 (polyethylene glycol with a molecular weight of 4000) and ε-caprolactone (mass ratio is 1: 24), under nitrogen protection, 100 degrees Celsius polymerization reaction for 5 hours, then set In vacuum at 160 degrees Celsius for 20 minutes, cooled to room temperature under the protection of nitrogen to obtain PCEC polymer, the relative molecular weight of the triblock polymer is 1 × 10 4 ~5×10 4 .
[0026] b. dissolving the PCEC polymer prepared according to step a in dichloromethane, and re-extracting with cold petroleum ether to obtain a purified PCEC polymer.
[0027] c. Dissolve the purified PCEC copolymer and lidocaine base prepared according to step b in ethyl acetate at 4 degrees Celsius, add F127 aqueou...
Embodiment 2
[0030] Example 2: Rapid transdermal administration method and effect verification of biodegradable nanoparticle gel with lidocaine as an example
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com