Production method of bromhexine hydrochloride
A technology of bromhexine hydrochloride and a production method, which is applied in the field of bromhexine hydrochloride raw material drug production, can solve problems such as synthesis reaction equipment being difficult to meet production needs, harsh synthesis reaction conditions, poor reaction quality and the like, and achieve sealing and environmental protection effects Good, simple structure, few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
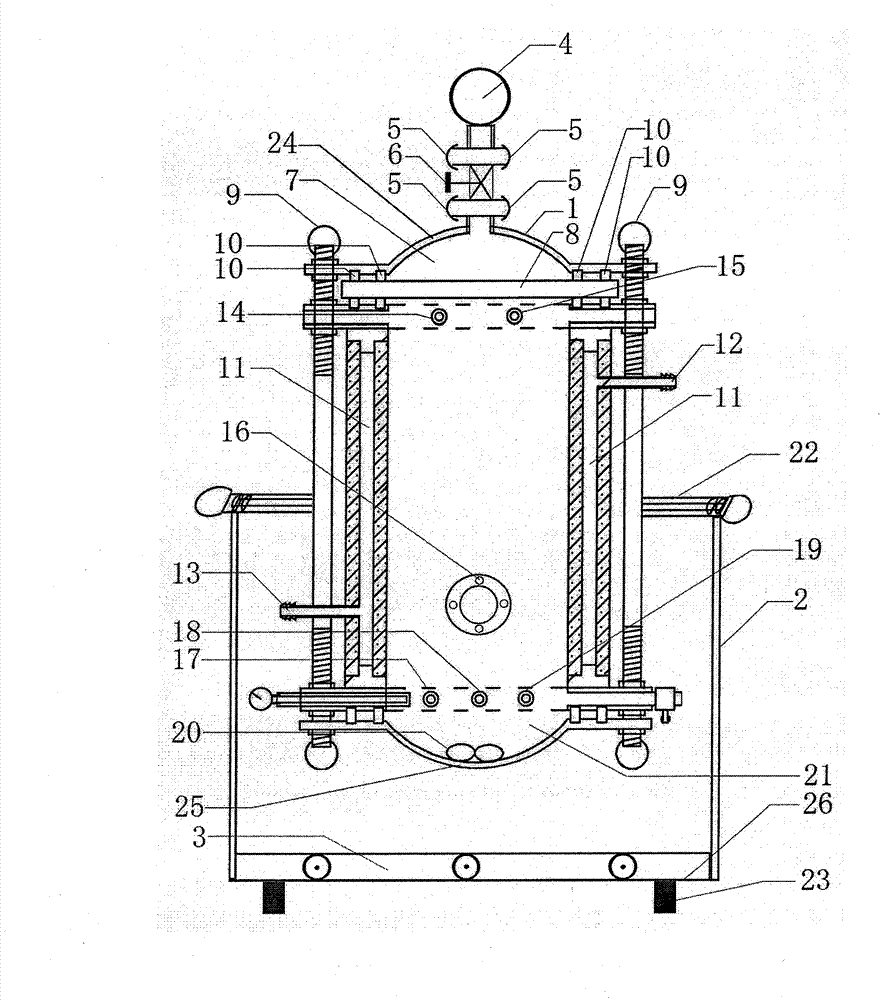
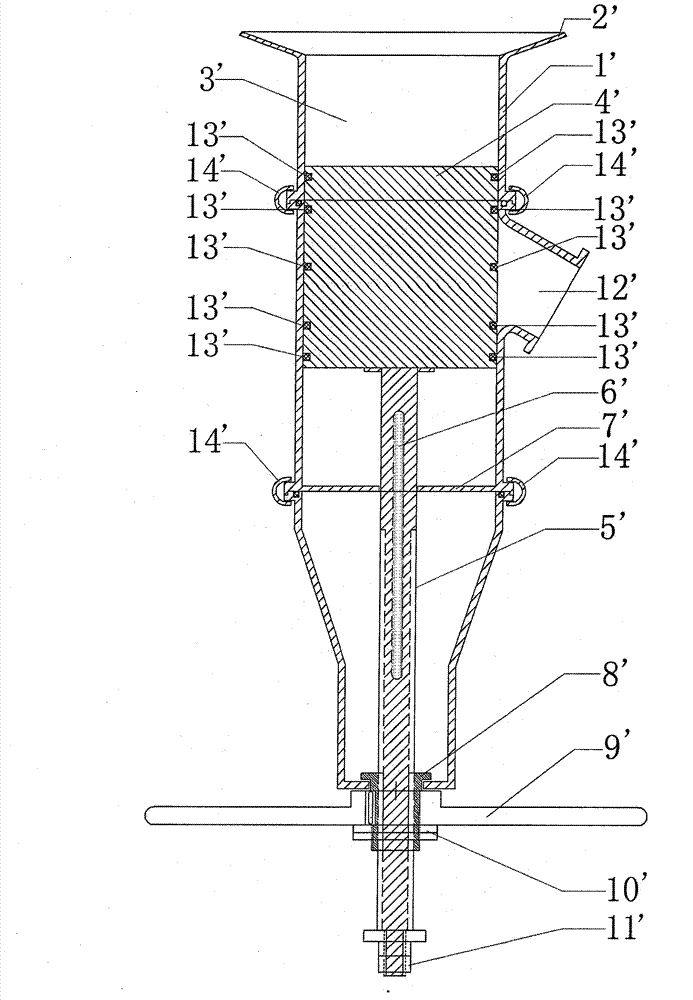
Image
Examples
Embodiment Construction
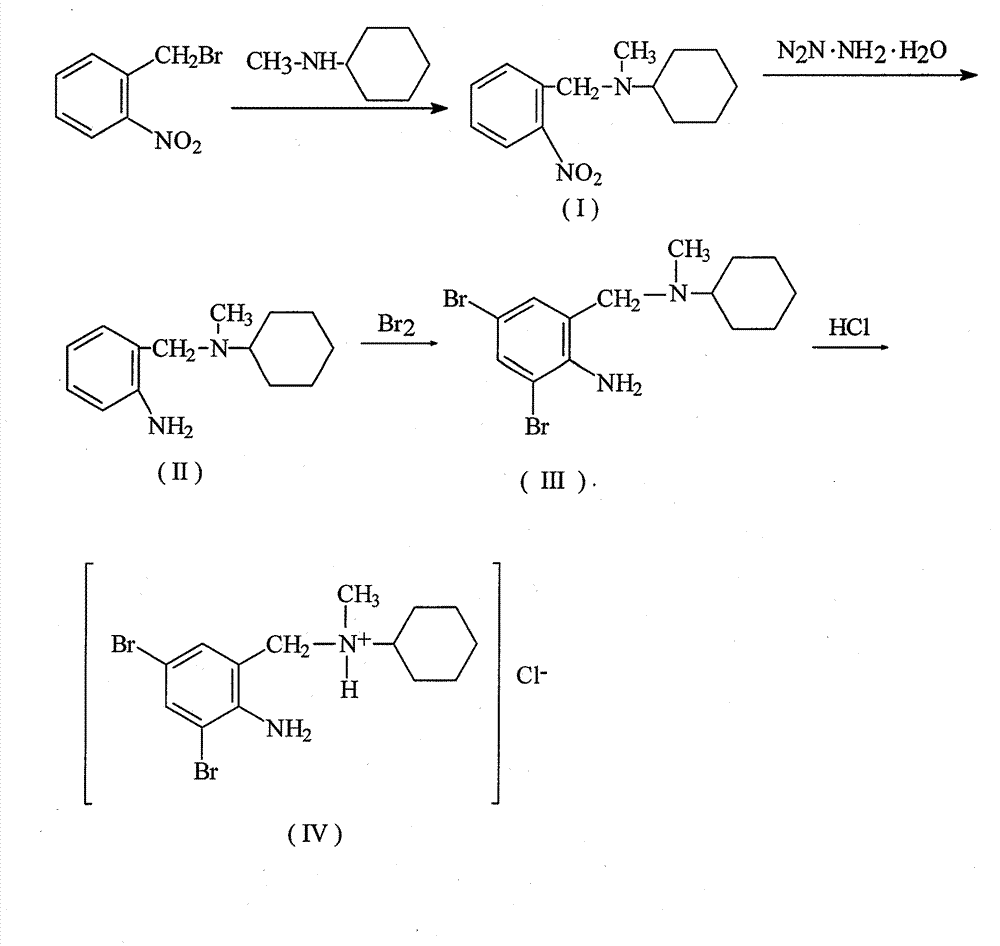
[0023] 1. Preparation of N-(2-nitrobenzyl)-N-methylcyclohexylamine (intermediate I)
[0024] Take 149g of 2-nitrobenzyl bromide, 80g of N-methylcyclohexylamine (about 99%), and 500ml of absolute ethanol in a multifunctional reactor equipped with a special bottom valve, and heat and reflux for 5h together. The reaction solution was evaporated to dryness under reduced pressure to remove ethanol. Add 100ml of water to dissolve the evaporated matter, add 2mol / L sodium hydroxide to adjust the pH to 10, extract 3 times with 50ml of chloroform, wash the organic layer 3 times with 30ml of water, dry with 50g of anhydrous sodium sulfate for more than 8 hours, filter and wash with 30ml of chloroform for 2 First, chloroform was distilled off under reduced pressure to obtain an oily residue, and then dried under reduced pressure at 116°C to 119°C and 8.0 Pa to obtain N-(2-nitrobenzyl)-N-methylcyclohexylamine oil (Intermediate I) 145 g. Yield 84.7%.
[0025] 2. Preparation of N-(2-amino...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com