Method for preparing cyclohexanol by using cyclohexene
A technology of cyclohexene and cyclohexanol, applied in the field of basic organic synthesis, can solve the problems of low yield of cyclohexanol, low reaction rate and high equipment cost, and achieve the effects of high yield, simple operation and low equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
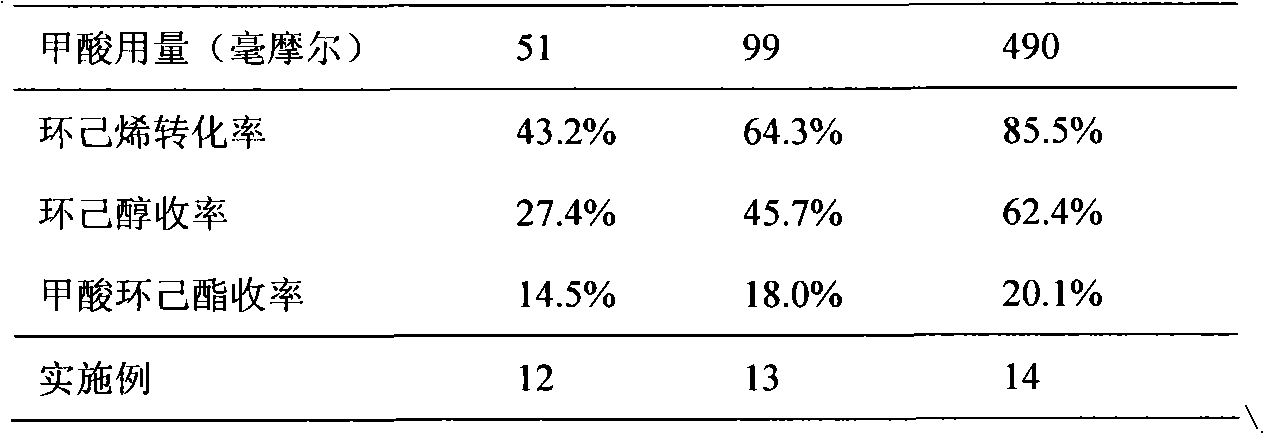
[0022] (1) Add 10 ml of cyclohexene (98.6 mmol), 11 ml of formic acid (294.0 mmol) and 2 g of HZSM-5 molecular sieve (0.2 g of molecular sieve / ml of cyclohexene) into a glass reactor, and stir rapidly after sealing , heated to 140°C, and reacted for 4 hours. A small amount of reaction liquid was taken out from the sampling valve and analyzed by gas chromatography. It can be seen that the conversion rate of cyclohexene was 75.3%, the yield of cyclohexanol was 1.2%, and the yield of cyclohexyl formate was 73.1%.
[0023] (2) Add 60 milliliters of deionized water into the reactor by using a metering pump, adjust the temperature to 180° C., and continue the reaction for 4 hours.
[0024] After the reaction, the solid catalyst was removed by filtration, and the composition of the organic phase in the filtrate was analyzed by gas chromatography. The conversion rate of cyclohexene was 81.2%, the yield of cyclohexanol was 60.5%, and the yield of cyclohexyl formate was 18.6%.
Embodiment 2~7
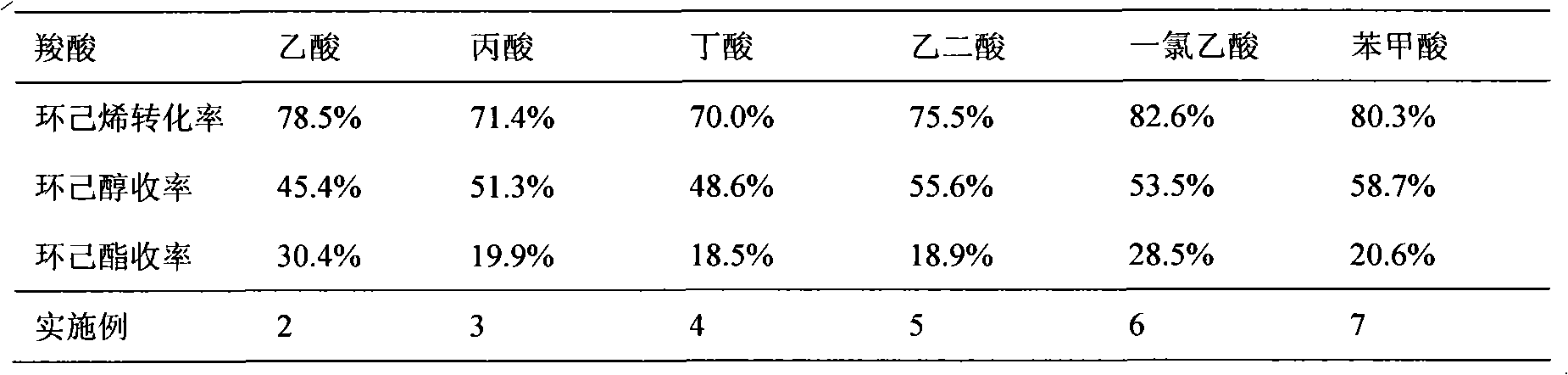
[0026] The amount of chemical reagents and the reaction steps are the same as in Example 1, but the type of carboxylic acid added is changed (the mol ratio of carboxylic acid to cyclohexene is the same as in Example 1), and the reaction results are shown in Table 1.
[0027] The impact of table 1 carboxylic acid species on the reaction of preparing cyclohexanol by cyclohexene
[0028]
Embodiment 8~11
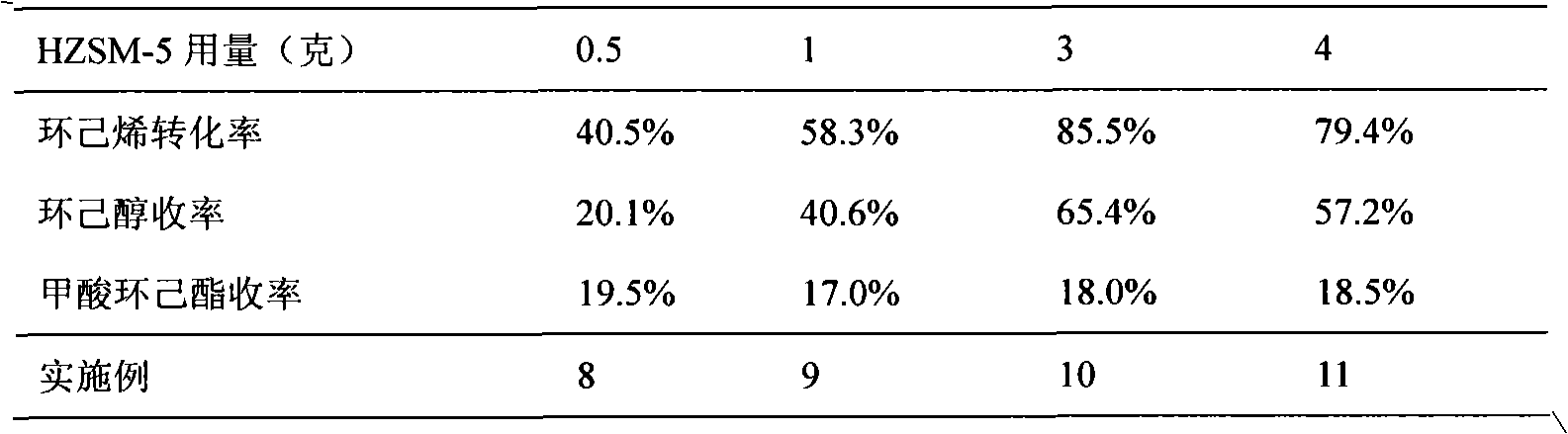
[0030] The amount of chemical reagents and the reaction steps are the same as in Example 1, but the amount of HZSM-5 added is changed, and the reaction results are shown in Table 2.
[0031] The impact of the amount of table 2HZSM-5 on the preparation of cyclohexanol by cyclohexene
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com