Mecobalamin sustained-release tablet and preparation method thereof
A sustained-release tablet, methylcobalamin technology, applied in the field of medicine, can solve the problems of no reports on methylcobalamin sustained-release preparations, difficulty in achieving effective release, inability to absorb methylcobalamin, etc., and achieve prescription reproducibility Good, good industrial production value, tablet smooth and beautiful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of Methylcobalamin Sustained Release Tablets
[0055] The prescription is (per 1000 tablets):
[0056] A: Methylcobalamin 1.5g
[0057] B: Micropowder silica gel 1.5g
[0058] C: Hypromellose (k4m) 20g
[0059] D: Carbomer (71G) 10g
[0060] E: microcrystalline cellulose (PH101) 65g
[0061] F: Magnesium stearate 1g
[0062] The materials A to F are crushed, passed through a 100-mesh sieve, and mixed uniformly to obtain a mixture; they are compressed into sustained-release tablets on a high-speed tablet machine. The above tablet was then coated with Opadry (OY-26528) opaque coating solution until the weight gain was 3%.
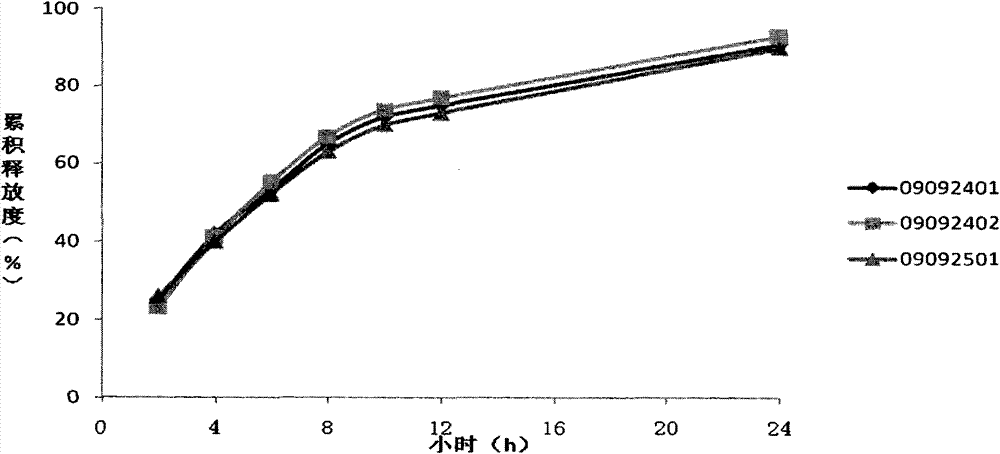
[0063] The results of tablet quality study showed that the average tablet weight was 102 mg, and the difference in tablet weight was less than 4.0%. Using 500 mL of phosphate buffer solution with pH 6.8 as the dissolution medium, the cumulative release rates of the three batches of methylcobalamin sustained-release tablets were all a...
Embodiment 2
[0065] Preparation of Methylcobalamin Sustained Release Tablets
[0066] The prescription is (per 1000 tablets):
[0067] A: Methylcobalamin 0.75g
[0068] B: Micropowder silica gel 1.5g
[0069] C: Hypromellose (k4m) 20g
[0070] D: Polyvinylpyrrolidone (K90) 20g
[0071] E: microcrystalline cellulose (PH101) 40g
[0072] F: Magnesium stearate (additional) 0.6g
[0073] G: Polyvinylpyrrolidone (K30) 5g
[0074] H: 95% ethanol 100g
[0075] Crush materials A to E, pass through a 100-mesh sieve, and mix uniformly to obtain a mixture; completely dissolve G: polyvinylpyrrolidone (K30) in H: 95% ethanol to prepare an adhesive; use this adhesive to prepare the mixture Granules, the drying temperature is controlled at 50°C; after the obtained granules are evenly mixed with material F, they are compressed into sustained-release tablets on a high-speed tablet press. The above tablet was then coated with Opadry (OY-26528) opaque coating solution until the weight gain was 3%.
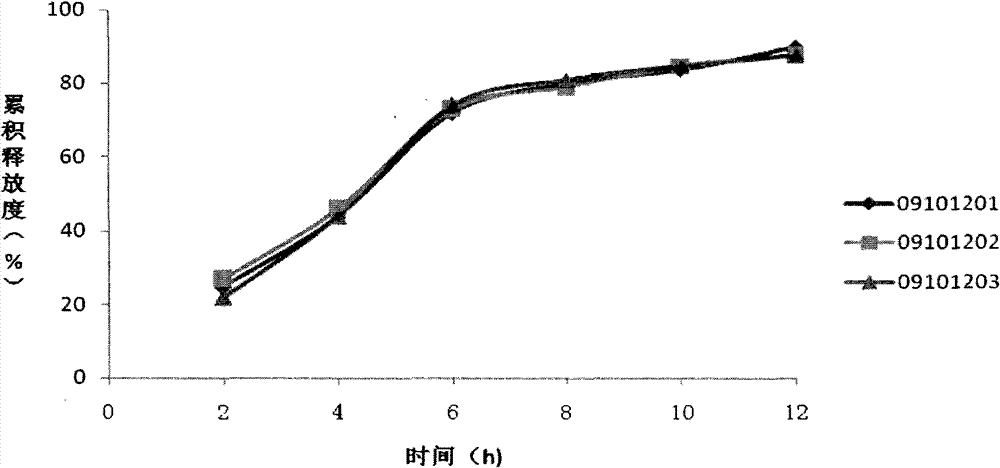
...
Embodiment 3
[0078] Preparation of Methylcobalamin Sustained Release Tablets
[0079] The prescription is (per 1000 tablets):
[0080] A: Methylcobalamin 1.5g
[0081] B: Micropowder silica gel 1.5g
[0082] C: Hypromellose (k4m) 25g
[0083] D: Hypromellose (k100m) 10g
[0084] E: microcrystalline cellulose (PH101) 20g
[0085] F: Mannitol 20g
[0086] G: Magnesium stearate (additional) 1g
[0087] H: Polyvinylpyrrolidone (K30) 5g
[0088] I: 95% ethanol 100g
[0089] Crush materials A to F, pass through a 100-mesh sieve, and mix uniformly to obtain a mixture; completely dissolve H: polyvinylpyrrolidone (K30) in I: 95% alcohol to prepare an adhesive; use this adhesive to prepare the mixture Granules, the drying temperature is controlled at 50°C; after the obtained granules are evenly mixed with material G, they are compressed into sustained-release tablets on a high-speed tablet press. The above tablet was then coated with Opadry (OY-26528) opaque coating solution until the weigh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com