Method for preparing light calcium carbonate and magnesium hydroxide from magnesium tailings
A technology of light calcium carbonate and magnesium hydroxide, which is applied to the removal of magnesium hydroxide, calcium carbonate/strontium/barium, solid waste, etc., can solve pollution and other problems, and achieve safe operation, low impurity MgO content, and good reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
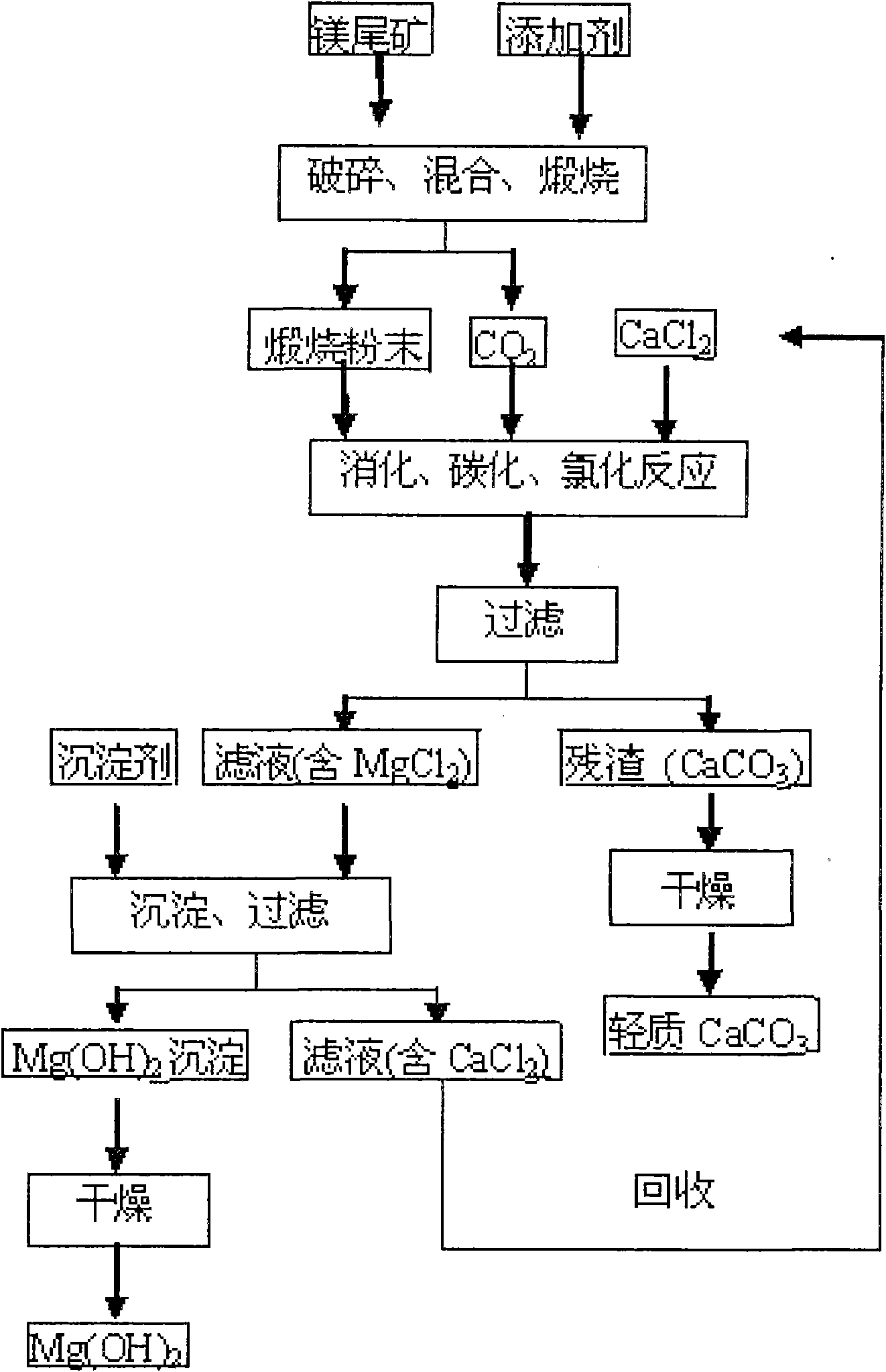
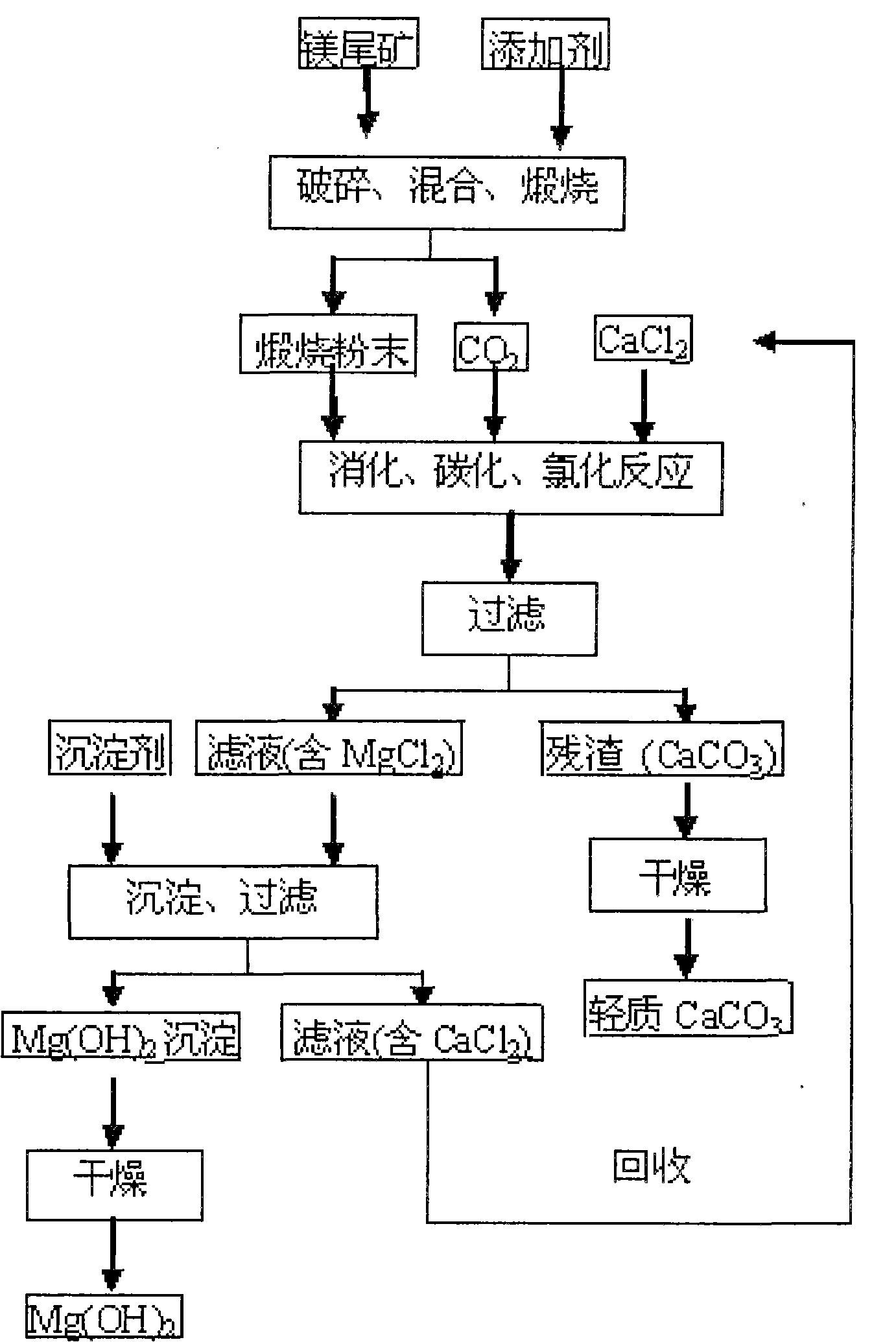
[0028] (1) The bulk magnesium tailings (calcium oxide content accounts for 24%) are crushed to obtain small pieces with a diameter of 16 to 19 mm, which are uniformly mixed with an additive of 1.4% by weight, wherein the additive is calcium chloride, and the additive The source of the calcium chloride solution is: the part of the calcium chloride solution mentioned in step (2) is obtained after evaporation and drying; then the mixture of the above-mentioned small magnesium tailings and additives is calcined at 670 ° C for 70 minutes to obtain calcined powder (mainly containing MgO and CaCO 3 ) and CO 2 , where CO 2 For subsequent digestion, carbonization and chlorination reactions;
[0029] (2) The calcined powder and calcium chloride solution were digested, carbonized, and chlorinated in a carbon dioxide atmosphere and stirred at 60°C for 120 minutes (the pH at the end of the reaction was 6.6-7.4), and then the solid-liquid separation was performed to obtain MgCl-containing...
Embodiment 2
[0033] (1) The bulk magnesium tailings (calcium oxide content accounts for 21%) are crushed to obtain small pieces with a diameter of 0.3 to 5 mm, which are uniformly mixed with an additive of 1.1% by weight, wherein the additive is calcium chloride, and the additive The source is: chemical reagent calcium chloride; then the mixture of the above-mentioned small magnesium tailings and additives is calcined at 840°C for 130min to obtain calcined powder (mainly containing MgO, CaO and CaCO 3 ) and CO 2 , where CO 2 For subsequent digestion, carbonization and chlorination reactions;
[0034] (2) The calcined powder and calcium chloride solution were digested, carbonized, and chlorinated for 80 minutes under the condition of stirring in a carbon dioxide atmosphere and 25°C (the pH at the end of the reaction was 6.6-7.4), and then the solid-liquid separation was carried out to obtain MgCl-containing 2 solution and CaCO 3 Precipitation; wherein the molar ratio of calcium chloride ...
Embodiment 3
[0038] (1) The bulk magnesium tailings (calcium oxide content accounts for 28%) are crushed to obtain small pieces with a diameter of 11 to 14 mm, which are uniformly mixed with an additive of 0.7% by weight, wherein the additive is magnesium chloride, and the source of the additive is It is: the part of the magnesium chloride solution mentioned in the step (2) is obtained after evaporation and drying; then the mixture of the above-mentioned small magnesium tailings and additives is calcined at 770°C for 30min to obtain calcined powder (mainly containing MgO, CaO and CaCO 3 ) and CO 2 , where CO 2 For subsequent digestion, carbonization and chlorination reactions;
[0039] (2) The calcined powder and calcium chloride solution were digested, carbonized, and chlorinated in a carbon dioxide atmosphere and stirred at 40°C for 100 minutes (the pH at the end of the reaction was 6.6-7.4), and then the solid-liquid separation was performed to obtain the MgCl-containing 2 solution an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com