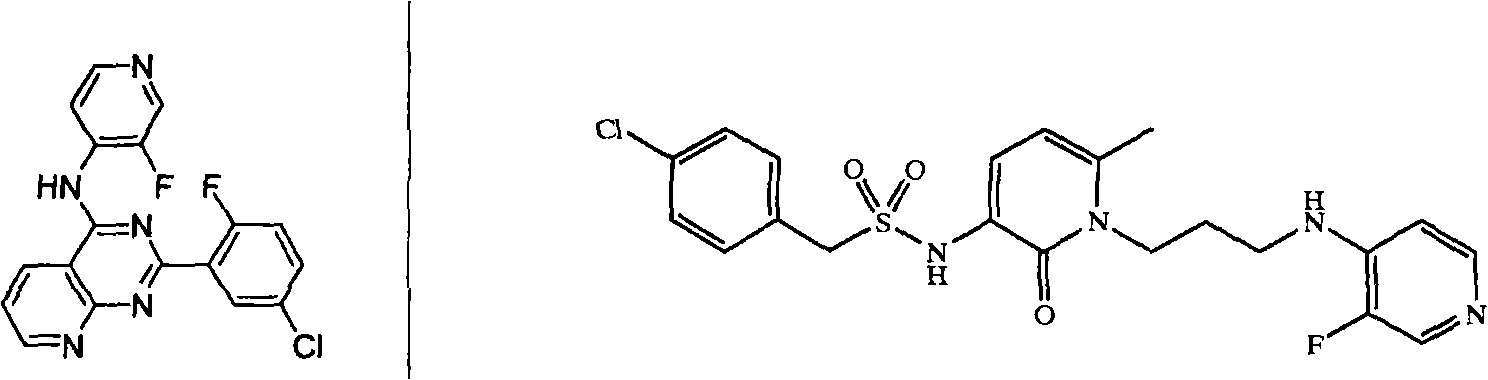
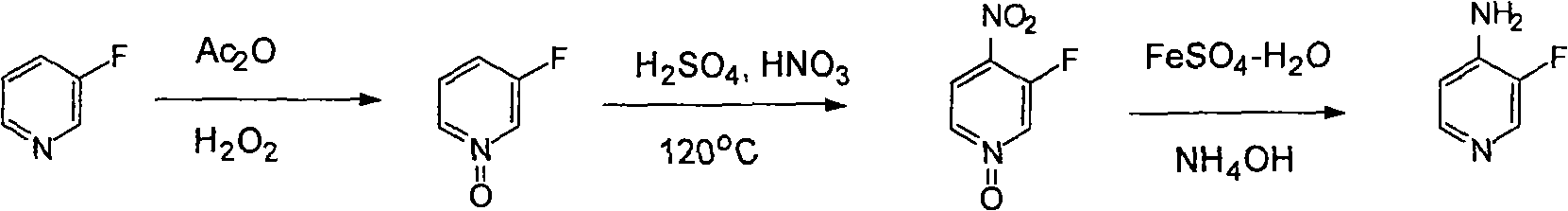
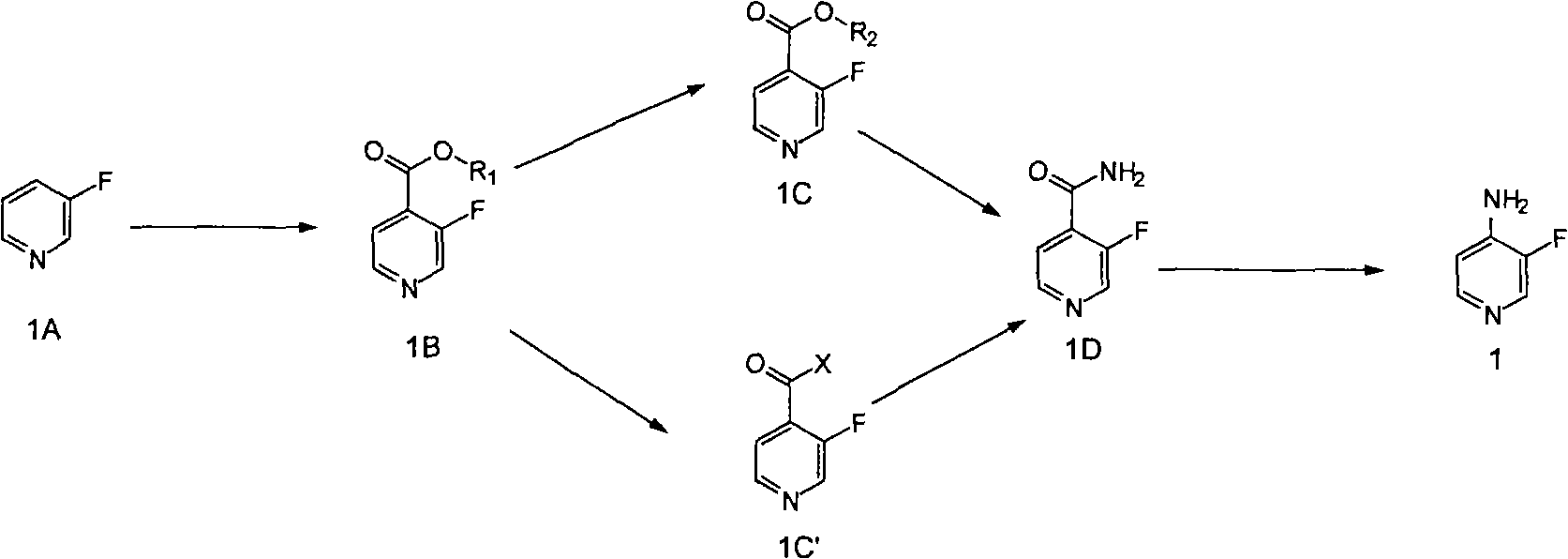
Synthesis method of 3-fluorine-4-aminopyridine
A technology of aminopyridine and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of easy explosion, low yield, difficult separation and purification, etc., and achieve the effect of high total yield, simple operation process and improved safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1). Preparation of 3-fluoro-4-pyridinecarboxylic acid
[0021] In a 250ml reaction flask, add 60ml of anhydrous tetrahydrofuran and 5.7g of diisopropylamine, cool to -25°C, add 22.5ml of 2.5M n-butyllithium dropwise, and keep the reaction solution at -30~-25°C After reacting for 1.5 hours, cool to -70°C, add 5.0g of 3-fluoropyridine, react at -70°C for 3 hours, slowly inject carbon dioxide gas, and warm the reaction solution to room temperature. Add 30ml of water dropwise into the reaction flask. Concentrate and distill off tetrahydrofuran. The pH of the reaction solution was adjusted to 3-5 with 2mol / l hydrochloric acid. At this point a large amount of solid appeared. The reaction was stirred at room temperature for 1 hour. Filter to get white solid powder. The powder was dried at 45° C. for 24 hours to obtain 6.5 g of product with a yield of 90% and a purity of 98%.
[0022] 1H-NMR (300MHz, D 2 O): 8.51(d, 2H), 8.42(dd, 1H), 7.57(t, 1H).
[0023] 2). Preparati...
Embodiment 2
[0049] 1). Preparation of methyl 3-fluoro-4-pyridinecarboxylate
[0050] In a 250ml reaction flask, add 480ml of anhydrous methanol and 71g of 3-fluoro-4-pyridinecarboxylic acid. Add 89.2 g of thionyl chloride dropwise to the reaction flask. After the dropwise addition, the reaction solution was heated to reflux. The reaction solution was refluxed for 24 hours. The reaction was cooled to room temperature. The solvent and excess thionyl chloride were distilled off to obtain a crude product. The crude product was washed with 200 ml of petroleum ether to obtain 71 g of methyl 3-fluoro-4-picolinate, with a yield of 91% and a purity of 96%.
[0051] 1H-NMR (300MHz, CD 3 OD): 9.08(d, 1H), 8.85(d, 1H), 8.34(t, 1H), 4.02(s, 3H).
[0052] 2) Preparation of ethyl 3-fluoro-4-pyridinecarboxylate
[0053] In a 250ml reaction flask, add 480ml of absolute ethanol and 71g of 3-fluoro-4-pyridinecarboxylic acid. Add 89.2 g of thionyl chloride dropwise to the reaction flask. After the d...
Embodiment 3
[0074] 1) Preparation of 3-fluoro-4-pyridinecarboxamide (optimum conditions)
[0075] In a 250ml reaction flask, add 50ml of anhydrous methanol and 5.0g of methyl 3-fluoro-4-picolinate. The reaction solution was cooled to -20~0°C. Slowly feed 15 g of ammonia gas into the reaction flask at -20 to 0 degrees. After the addition of ammonia gas was completed, the reaction solution was warmed up to room temperature and stirred at room temperature for 6 hours. Ammonia and solvent were evaporated under reduced pressure to obtain 4.1 g of a light yellow solid product with a yield of 91% and a purity of 96%.
[0076] 1H-NMR (300MHz, CDCl 3 ): 8.60 (m, 2H), 7.94 (t, 1H), 6.67 (br s, 1H), 6.41 (br s, 1H).
[0077] 2) Preparation of 3-fluoro-4-pyridinecarboxamide (tetrahydrofuran)
[0078] In a 250ml reaction flask, add 50ml tetrahydrofuran and 5.0g methyl 3-fluoro-4-picolinate. The reaction solution was cooled to -20~0°C. Slowly feed 15 g of ammonia gas into the reaction flask at -20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com